Testing a Machine Learning-Based Adaptive Motivational System for Socioeconomically Disadvantaged Smokers (Adapt2Quit): Protocol for a Randomized Controlled Trial
- PMID: 40239194
- PMCID: PMC12044314
- DOI: 10.2196/63693
Testing a Machine Learning-Based Adaptive Motivational System for Socioeconomically Disadvantaged Smokers (Adapt2Quit): Protocol for a Randomized Controlled Trial
Erratum in
-
Correction: Testing a Machine Learning-Based Adaptive Motivational System for Socioeconomically Disadvantaged Smokers (Adapt2Quit): Protocol for a Randomized Controlled Trial.JMIR Res Protoc. 2025 Dec 9;14:e79873. doi: 10.2196/79873. JMIR Res Protoc. 2025. PMID: 41364920 Free PMC article.
Abstract
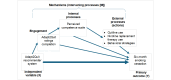
Background: Individuals who are socioeconomically disadvantaged have high smoking rates and face barriers to participating in smoking cessation interventions. Computer-tailored health communication, which is focused on finding the most relevant messages for an individual, has been shown to promote behavior change. We developed a machine learning approach (the Adapt2Quit recommender system), and our pilot work demonstrated the potential to increase message relevance and smoking cessation effectiveness among individuals who are socioeconomically disadvantaged.
Objective: This study protocol describes our randomized controlled trial to test whether the Adapt2Quit recommender system will increase smoking cessation among individuals from socioeconomically disadvantaged backgrounds who smoke.
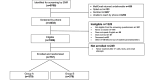
Methods: Individuals from socioeconomically disadvantaged backgrounds who smoke were identified based on insurance tied to low income or from clinical settings (eg, community health centers) that provide care for low-income patients. They received text messages from the Adapt2Quit recommender system for 6 months. Participants received daily text messages for the first 30 days and every 14 days until the end of the study. Intervention participants also received biweekly texting facilitation messages, that is, text messages asking participants to respond (yes or no) if they were interested in being referred to the quitline. Interested participants were then actively referred to the quitline by study staff. Intervention participants also received biweekly text messages assessing their current smoking status. Control participants did not receive the recommender messages but received the biweekly texting facilitation and smoking status assessment messages. Our primary outcome is the 7-day point-prevalence smoking cessation at 6 months, verified by carbon monoxide testing. We will use an inverse probability weighting approach to test our primary outcome. This involves using a logistic regression model to predict nonmissingness, calculating the inverse probability of nonmissingness, and using it as a weight in a logistic regression model to compare cessation rates between the two groups.
Results: The Adapt2Quit study was funded in April 2020 and is still ongoing. We have completed the recruitment of individuals (N=757 participants). The 6-month follow-up of all participants was completed in November 2024. The sample consists of 64% (486/757) female participants, 35% (265/757) Black or African American individuals, 51.1% (387/757) White individuals, and 16% (121/757) Hispanic or Latino individuals. In total, 52.6% (398/757) of participants reported having a high school education or being a high school graduate; 70% (529/757) smoked their first cigarette within 30 minutes of waking, and half (379/757, 50%) had stopped smoking for at least one day in the past year. Moreover, 16.6% (126/757) had called the quitline before study participation.
Conclusions: We have recruited a diverse sample of individuals who are socioeconomically disadvantaged and designed a rigorous protocol to evaluate the Adapt2Quit recommender system. Future papers will present our main analysis of the trial.
Trial registration: ClinicalTrials.gov NCT04720625; https://clinicaltrials.gov/study/NCT04720625.
International registered report identifier (irrid): DERR1-10.2196/63693.
Keywords: mHealth; machine learning; smoking cessation; socioeconomically disadvantaged, biochemical verification.
©Ariana Kamberi, Benjamin Weitz, Julie Flahive, Julianna Eve, Reem Najjar, Tara Liaghat, Daniel Ford, Peter Lindenauer, Sharina Person, Thomas K Houston, Megan E Gauvey-Kern, Jackie Lobien, Rajani S Sadasivam. Originally published in JMIR Research Protocols (https://www.researchprotocols.org), 16.04.2025.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures
References
-
- Smoking cessation. A report of the surgeon general. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health. 2020. [2025-04-04]. https://www.hhs.gov/sites/default/files/2020-cessation-sgr-full-report.pdf .
-
- Smoking and tobacco use. Centers for Disease Control and Prevention. [2015-06-18]. https://www.cdc.gov/tobacco/index.html .
-
- PAR-16-202: improving smoking cessation in socioeconomically disadvantaged populations via scalable interventions (R01) National Institutes of Health. [2016-10-01]. https://grants.nih.gov/grants/guide/pa-files/PAR-16-202.html .
-
- Tobacco control research priorities for the next decade: working group recommendations for 2016 – 2025. National Cancer Institute Division of Extramural Activities. [2025-04-04]. https://deainfo.nci.nih.gov/advisory/bsa/archive/0316/1115ResearchPriori... .
-
- U.S. National Cancer Institute A socioecological approach to addressing tobacco-related health disparities. U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute. 2017. [2025-04-04]. https://cancercontrol.cancer.gov/brp/tcrb/monographs/monograph-22 .
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous