Modulatory effects of polyherbal mixture on the immuno-antioxidant capacity and intestinal health of chicks infected with Escherichia coli O78
- PMID: 40239311
- PMCID: PMC12032338
- DOI: 10.1016/j.psj.2025.105156
Modulatory effects of polyherbal mixture on the immuno-antioxidant capacity and intestinal health of chicks infected with Escherichia coli O78
Abstract
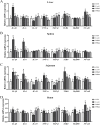
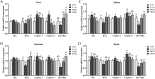
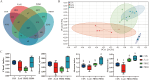
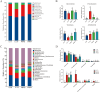
A total of 180 one-day-old white-feathered broiler chicks were selected and randomly divided into 4 treatments, namely the control group (CON), Escherichia coli groups (E. coli), 2 g/kg polyherbal mixture group (PHM2), and the 4 g/kg polyherbal mixture group (PHM4). The CON and E. coli groups were fed a basal diet, while the PHM2 and PHM4 groups were fed the basal diet supplemented with 2 g/kg and 4 g/kg PHM, respectively. Each group had 3 replicates, with 15 broilers per replicate. On day 17 of the experiment, broilers in the E. coli, PHM2, and PHM4 groups were intraperitoneally injected with 0.8 mL of 1 × 108 CFU/mL of E. coli O78. Broilers in the control group received an equivalent volume of saline. Chicks were euthanized 48 h postinjection for collecting serum, liver, spleen, jejunum, ileum, ileal mucosa, and cecal contents. Our results showed that PHM significantly reversed the weight loss and decreased the diarrhea rate and the mortality of chicks caused by E. coli infection (P < 0.05). In the serum of chicks infected with E. coli, PHM significantly enhanced the antioxidant capacity (P < 0.05), increased the levels of immunoglobulins and anti-inflammatory cytokines (P < 0.05), and decreased the concentrations of proinflammatory cytokines (P < 0.05). Meanwhile, PHM also promoted the mRNA expression of antioxidant-related genes and decreased the expression of proinflammatory cytokines and apoptosis-related genes in the liver, spleen, jejunum, and ileum (P < 0.05). In addition, PHM repaired the intestinal barrier and injury to further reduce the serum concentrations of d-lactate (DAO) and lipopolysaccharide (LPS) (P < 0.05). More importantly, PHM significantly regulated the composition of cecal microbiota, especially by up-regulating the relative abundance of beneficial bacteria, including Faecalibacterium, Bacteroides, Butyricicoccus, and Lactobacillus, and down-regulating the relative abundance of pathogenic bacteria, including Enterococcus, Escherichia, and Shigella (P < 0.05). These beneficial bacteria were significantly positively correlated with antioxidant capacity and intestinal barrier function, while pathogenic bacteria were significantly positively correlated with proinflammatory cytokines (P < 0.05). In conclusion, PHM may be a potential preventive strategy for E. coli-infected poultry, which is closely related to its modulation of gut microbiota.
Keywords: Chick; Escherichia coli O78; Immune response; Polyherbal mixture; intestinal health.
Copyright © 2025. Published by Elsevier Inc.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





Similar articles
-
Influence of purslane extract on immuno-antioxidant status, intestinal barrier, and microbiota of chicks after experimental infection with Escherichia coli O78.Poult Sci. 2025 Jun;104(6):105106. doi: 10.1016/j.psj.2025.105106. Epub 2025 Mar 29. Poult Sci. 2025. PMID: 40245541 Free PMC article.
-
Chlorogenic acid mitigates avian pathogenic Escherichia coli-induced intestinal barrier damage in broiler chickens via anti-inflammatory and antioxidant effects.Poult Sci. 2025 May;104(5):105005. doi: 10.1016/j.psj.2025.105005. Epub 2025 Mar 7. Poult Sci. 2025. PMID: 40086255 Free PMC article.
-
Effects of dietary polyherbal mixtures on growth performance, antioxidant capacity, immune function and jejunal health of yellow-feathered broilers.Poult Sci. 2023 Jul;102(7):102714. doi: 10.1016/j.psj.2023.102714. Epub 2023 Apr 12. Poult Sci. 2023. PMID: 37172360 Free PMC article.
-
Dietary supplementary with ellagic acid improves the intestinal barrier function and flora structure of broiler chicken challenged with E. coli K88.Poult Sci. 2024 Dec;103(12):104429. doi: 10.1016/j.psj.2024.104429. Epub 2024 Oct 13. Poult Sci. 2024. PMID: 39461273 Free PMC article.
-
Effects of Dietary Supplementation of Probiotic Mix and Prebiotic on Growth Performance, Cecal Microbiota Composition, and Protection Against Escherichia coli O78 in Broiler Chickens.Probiotics Antimicrob Proteins. 2019 Sep;11(3):981-989. doi: 10.1007/s12602-018-9459-y. Probiotics Antimicrob Proteins. 2019. PMID: 30187428
Cited by
-
Selenium-enriched Lactobacillus coryniformis H8 alleviates LPS-induced jejunal injury in chicks by modulating antioxidant activity, inflammation, selenoprotein genes, and gut microbiota.Poult Sci. 2025 Aug 13;104(11):105675. doi: 10.1016/j.psj.2025.105675. Online ahead of print. Poult Sci. 2025. PMID: 40840291 Free PMC article.
References
-
- Abo Ghanima M., Aljahdali N., Abuljadayel D., Shafi M., Qadhi A., Elgammal M., Mohamed L. Effects of dietary supplementation of Amla, Chicory and Leek extracts on growth performance, immunity and blood biochemical parameters of broilers. Ital. J. Anim. Sci. 2023;22(1):24–34.
-
- Ahmed L., Al-Massri K. Gut microbiota modulation for therapeutic management of various diseases: a new perspective using stem cell therapy. Curr. Mol. Pharmacol. 2022;15 - PubMed
-
- Awad N., Abd E.M., Hashem Y.M., Erfan A.M., Abdelrahman B.A., Mahmoud H.I. Impact of single and mixed infections with Escherichia coli and Mycoplasma gallisepticum on Newcastle disease virus vaccine performance in broiler chickens: an in vivo perspective. J. Appl. Microbiol. 2019;127(2):396–405. - PubMed
-
- Ben Y.H., Trabelsi I.., Arous F., Garcia-Vela S., Torres C., Ben S.K. Detection of linezolid and vancomycin resistant Enterococcus isolates collected from healthy chicken caecum. J. Appl. Microbiol. 2024;135(2) - PubMed
-
- Cai Y., Kang Y. Gut microbiota and metabolites in diabetic retinopathy: insights into pathogenesis for novel therapeutic strategies. Biomed. Pharmacother. 2023;164 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

