Phase 2 trial of perioperative chemo-immunotherapy for gastro-esophageal adenocarcinoma: The role of M2 macrophage landscape in predicting response
- PMID: 40239627
- PMCID: PMC12047487
- DOI: 10.1016/j.xcrm.2025.102045
Phase 2 trial of perioperative chemo-immunotherapy for gastro-esophageal adenocarcinoma: The role of M2 macrophage landscape in predicting response
Abstract
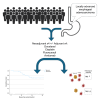
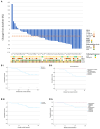
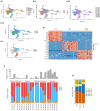
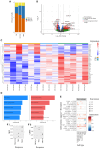
We present the clinical results of a phase 2 trial combining neoadjuvant docetaxel, cisplatin, 5 Flourouracil, and the PD-L1 inhibitor avelumab in locally advanced gastro-esophageal adenocarcinoma (GEA). Fifty-one patients receive neoadjuvant therapy with 50 proceeding to surgery. Grade 3-4 adverse events occur in 40%; complete/major pathological response is found in 7/50 (14%) and 9/50 (18%), with 2-year disease-free survival of 67.5%. There is no correlation between tumor regression and PD-L1 or mismatch repair (MMR) status. Multiplex immunohistochemistry and longitudinal single-cell transcriptomic profiling reveal alterations in certain innate immune cell populations, particularly noting an M2-tumor-associated macrophage (M2-TAM) proliferation in non-responding tumors. These findings describe the effective nature of this treatment regimen for GEA and reveal associated features of the inflammatory milieux associated with response to chemo-immunotherapy. The specific character of the inflammatory environment in non-responders may, in the future, help personalize treatment. This study was registered at ClinicalTrials.gov (NCT03288350).
Keywords: chemotherapy; gastroesophageal adenocarcinoma; immune microenvironment; immunotherapy; pathologic response; single cell transcriptomics; spatial proteomics; surgery; tumor associated macrophage.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests T.A. has received research funding from EMD Serono and consultancy fees for BMS, Roche, Merck, Novartis, and Tiaho. The rest of the authors declare no competing interests.
Figures


References
-
- Boonstra J.J., Kok T.C., Wijnhoven B.P., van Heijl M., van Berge Henegouwen M.I., Ten Kate F.J., Siersema P.D., Dinjens W.N., van Lanschot J.J., Tilanus H.W., van der Gaast A. Chemotherapy followed by surgery versus surgery alone in patients with resectable oesophageal squamous cell carcinoma: long-term results of a randomized controlled trial. BMC Cancer. 2011;11:181. doi: 10.1186/1471-2407-11-181. - DOI - PMC - PubMed
-
- van Hagen P., Hulshof M.C.C.M., van Lanschot J.J.B., Steyerberg E.W., van Berge Henegouwen M.I., Wijnhoven B.P.L., Richel D.J., Nieuwenhuijzen G.A.P., Hospers G.A.P., Bonenkamp J.J., et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N. Engl. J. Med. 2012;366:2074–2084. doi: 10.1056/NEJMoa1112088. - DOI - PubMed
-
- Al-Batran S.E., Homann N., Pauligk C., Goetze T.O., Meiler J., Kasper S., Kopp H.G., Mayer F., Haag G.M., Luley K., et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet. 2019;393:1948–1957. doi: 10.1016/S0140-6736(18)32557-1. - DOI - PubMed
-
- Möhring C., Mańczak A., Timotheou A., Sadeghlar F., Zhou T., Mahn R., Monin M.B., Toma M., Feldmann G., Brossart P., Köksal M. Perioperative therapy with FLOT4 significantly increases survival in patients with gastroesophageal and gastric cancer in a large real-world cohort. Int. J. Cancer. 2023;153:609–622. doi: 10.1002/ijc.34511. - DOI - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

