Animal models for the evaluation of retinal stem cell therapies
- PMID: 40239758
- PMCID: PMC12145155
- DOI: 10.1016/j.preteyeres.2025.101356
Animal models for the evaluation of retinal stem cell therapies
Abstract
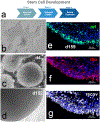
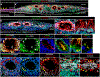
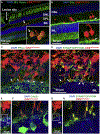
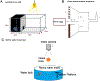
Retinal degeneration (RD) diseases leading to severe vision loss can affect photoreceptors (PRs) that are responsible for phototransduction, or retinal pigmented epithelium (RPE) providing support for PRs. Human pluripotent stem cell (hPSC)-based therapies are a potential approach for restoration of retinal structure in patients with currently incurable RD diseases. Currently, there are two targeted hPSC therapeutics: PR rescue and PR replacement. PR rescue involves the transplantation of RPE or other neural progenitors into the subretinal space to slow down or prevent further RD. RPE transplantation plays a critical role in preserving photoreceptors by providing trophic support and maintaining retinal integrity, particularly in diseases like age-related macular degeneration (AMD). Advances in RPE transplantation methods, such as polarized monolayer cultures and scaffold-based approaches, have shown promise in enhancing graft survival and integration. However, limitations include inconsistent integration, variable neurotrophic factor secretion, and immune rejection risks in non-autologous transplants. In PR replacement, stem cell-derived photoreceptor-like cells or photoreceptor progenitors (PRP) obtained are transplanted into the eye. While PRPs are commonly obtained from retinal organoids (ROs), alternative sources, such as early differentiation stages or direct differentiation protocols, are also utilized to enhance the efficiency and scalability of PRP generation. Challenges include achieving proper integration, forming outer segments, rosette formation, and avoiding immune rejection or tumorigenicity. Various animal models that simulate human RD diseases are being used for establishing surgical feasibility, graft survival and visual functional recovery but fail to replicate clinical immune challenges. Rodent models lack macula-like structures and have limited reliability in detecting subtle functional changes, while larger animal models pose ethical, logistical, and financial challenges. Immunocompromised models have been developed for minimizing xenograft issues. Visual functional testing for efficacy includes optokinetic testing (OKN), electroretinography (ERG), and electrophysiological recordings from the retina and brain. These tests often fail to capture the complexity of human visual recovery, highlighting the need for advanced models and improved functional testing techniques. This review aims to aggregate current knowledge about approaches to stem cell transplantation, requirements of animal models chosen for validating vision benefits of transplantation studies, advantages of using specific disease models and their limitations. While promising strides have been made, addressing these limitations remains essential for translating stem cell-based therapies into clinical success.
Keywords: Pluripotent stem cells; Retinal degeneration; Retinal disease models; Retinal organoids; Vision testing.
Copyright © 2025 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests BBT, DRN, MR, AKH, and TT have no financial interests to disclose. MJS (co-inventor): patent # 12,247,220: “3D printed micro-millifluidic bioreactors for long-term retinal organoid maintenance”, issued in March 2025.
Figures

References
-
- Aboualizadeh E, Phillips MJ, McGregor JE, DiLoreto DA Jr., Strazzeri JM, Dhakal KR, Bateman B, Jager LD, Nilles KL, Stuedemann SA, Ludwig AL, Hunter JJ, Merigan WH, Gamm DM, Williams DR, 2020. Imaging transplanted photoreceptors in living nonhuman primates with single-cell resolution. Stem Cell Rep. 15, 482–497. 10.1016/j.stemcr.2020.06.019. - DOI - PMC - PubMed
-
- Ahmed F, Rajendran Nair DS, Thomas BB, 2022. A new optokinetic testing method to measure rat vision. J. Vis. Exp 185. 10.3791/63357, 10.3791/63357. - DOI - DOI - PMC - PubMed
-
- Ail D, Nava D, Hwang IP, Brazhnikova E, Nouvel-Jaillard C, Dentel A, Joffrois C, Rousseau L, Degardin J, Bertin S, Sahel JA, Goureau O, Picaud S, Dalkara D, 2023. Inducible nonhuman primate models of retinal degeneration for testing end-stage therapies. Sci. Adv 9, eadg8163. 10.1126/sciadv.adg8163. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

