A Critical Role for the Mitochondrial Pyruvate Carrier in Hepatic Stellate Cell Activation
- PMID: 40239806
- PMCID: PMC12166444
- DOI: 10.1016/j.jcmgh.2025.101517
A Critical Role for the Mitochondrial Pyruvate Carrier in Hepatic Stellate Cell Activation
Abstract
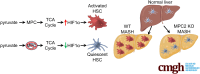
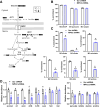
Background & aims: Hepatic stellate cells (HSCs) are non-parenchymal cells of the liver that produce the extracellular matrix that forms fibrotic lesions in chronic liver disease, including metabolic dysfunction-associated steatohepatitis (MASH). The mitochondrial pyruvate carrier (MPC) catalyzes the transport of pyruvate from the cytosol into the mitochondrial matrix, which is a critical step in pyruvate metabolism. An MPC inhibitor has shown promise as a novel therapeutic for MASH and HSC activation, but a mechanistic understanding of the direct effects of MPC inhibition on HSC activation is lacking.
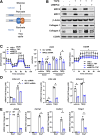
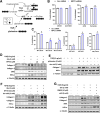
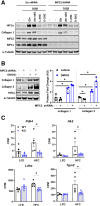
Methods: Stable lines of LX2 cells expressing short hairpin RNA against MPC2 were established and examined in a series of studies to assess HSC metabolism and activation. Mice with conditional, HSC-specific MPC2 deletion were generated and their phenotypes assessed in the context of diets that cause hepatic steatosis, injury, and early-stage fibrosis.
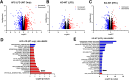
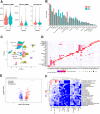
Results: Genetic suppression of MPC activity markedly decreased expression of markers of HSC activation in vitro. MPC knockdown reduced the abundance of several intermediates of the tricarboxylic acid cycle and attenuated HSC activation by suppressing hypoxia inducible factor-1α signaling. Supplementing alpha-ketoglutarate to replenish the tricarboxylic acid cycle intermediates was sufficient to overcome the effects of MPC inhibition on hypoxia inducible factor-1α and HSC activation. On high-fat diets, mice with HSC-specific MPC deletion exhibited reduced circulating transaminases, numbers of HSCs, and hepatic expression of markers of HSC activation and inflammation compared with wild-type mice.
Conclusions: These data suggest that MPC inhibition modulates HSC metabolism to attenuate activation and illuminate mechanisms by which MPC inhibitors could prove therapeutically beneficial for treating MASH.
Keywords: Collagen; Fibrosis; HIF1alpha; MASLD; TCA Cycle.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Figures





References
-
- Younossi Z.M., Koenig A.B., Abdelatif D., et al. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. - PubMed
-
- Younossi Z., Anstee Q.M., Marietti M., et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11–20. - PubMed
-
- Taylor R.S., Taylor R.J., Bayliss S., et al. Association between fibrosis stage and outcomes of patients with nonalcoholic fatty liver disease: a systematic review and meta-analysis. Gastroenterology. 2020;158:1611–1625.e12. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

