Role of amino acids in the regulation of hepatic gluconeogenesis and lipogenesis in metabolic dysfunctionassociated steatotic liver disease
- PMID: 40239968
- PMCID: PMC12260646
- DOI: 10.3350/cmh.2025.0048
Role of amino acids in the regulation of hepatic gluconeogenesis and lipogenesis in metabolic dysfunctionassociated steatotic liver disease
Abstract
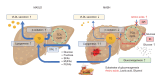
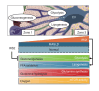
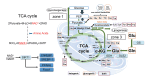
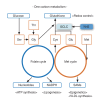
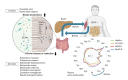
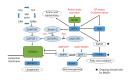
Metabolic dysfunction-associated steatotic liver disease (MASLD) and its relatively advanced form, metabolic dysfunction-associated steatohepatitis (MASH), are becoming increasingly prevalent worldwide, making their prevention and management an urgent global health priority. Central to their development are key metabolic defects, including abnormal concentrations of monosaccharides, fatty acids, and amino acids, but the complex relationships between these substances within the hepatic microenvironment remain only partially understood. Dysregulated glucose metabolism and selective insulin resistance (IR) promote hepatic gluconeogenesis, glycolysis, and de novo lipogenesis; and excessive concentrations of free fatty acids from the diet and adipose tissue drive steatosis. Emerging evidence also implies that amino acid metabolism affects mitochondrial function and redox balance. Dysfunctional mitochondrial oxidative phosphorylation and the associated increase in reactive oxygen species production further exacerbate the cellular stress, inflammation, and fibrosis. However, compared with monosaccharide and fatty acid metabolism, the role of amino acid metabolism in MASLD/MASH remains less well understood. A better understanding of the role of such metabolic dysfunction in liver pathobiology should aid the identification of more useful biomarkers and precision therapies for MASLD/MASH.
Keywords: Amino acid; Fatty acid; Gluconeogenesis; Glucose; Lipogenesis.
Conflict of interest statement
The authors have no conflicts to disclose.
Figures

Similar articles
-
Metabolic dysfunction-associated steatotic liver disease: A story of muscle and mass.World J Gastroenterol. 2025 May 28;31(20):105346. doi: 10.3748/wjg.v31.i20.105346. World J Gastroenterol. 2025. PMID: 40495947 Free PMC article.
-
Integrative metabolism in MASLD and MASH: Pathophysiology and emerging mechanisms.J Hepatol. 2025 Aug;83(2):584-595. doi: 10.1016/j.jhep.2025.02.033. Epub 2025 Mar 1. J Hepatol. 2025. PMID: 40032040 Review.
-
Induction of Fructose Mediated De Novo Lipogenesis Coexists with the Upregulation of Mitochondrial Oxidative Function in Mice Livers.J Nutr. 2025 Jun;155(6):1768-1781. doi: 10.1016/j.tjnut.2025.04.030. Epub 2025 May 5. J Nutr. 2025. PMID: 40334788 Free PMC article.
-
Inappropriate Diet Exacerbates Metabolic Dysfunction-Associated Steatotic Liver Disease via Abdominal Obesity.Nutrients. 2024 Dec 5;16(23):4208. doi: 10.3390/nu16234208. Nutrients. 2024. PMID: 39683601 Free PMC article.
-
Cinnabarinic acid protects against metabolic dysfunction-associated steatohepatitis by activating aryl hydrocarbon receptor-dependent AMPK signaling.Am J Physiol Gastrointest Liver Physiol. 2025 Apr 1;328(4):G433-G447. doi: 10.1152/ajpgi.00337.2024. Epub 2025 Mar 10. Am J Physiol Gastrointest Liver Physiol. 2025. PMID: 40062565 Free PMC article.
Cited by
-
Food Nutrients and Bioactive Compounds for Managing Metabolic Dysfunction-Associated Steatotic Liver Disease: A Comprehensive Review.Nutrients. 2025 Jul 3;17(13):2211. doi: 10.3390/nu17132211. Nutrients. 2025. PMID: 40647314 Free PMC article. Review.
-
Current Data on the Role of Amino Acids in the Management of Obesity in Children and Adolescents.Int J Mol Sci. 2025 Jul 24;26(15):7129. doi: 10.3390/ijms26157129. Int J Mol Sci. 2025. PMID: 40806262 Free PMC article. Review.
References
-
- Zhao Q, Deng Y. Comparison of mortality outcomes in individuals with MASLD and/or MAFLD. J Hepatol. 2024;80:e62–e64. - PubMed
-
- Mantovani A, Csermely A, Petracca G, Beatrice G, Corey KE, Simon TG, et al. Non-alcoholic fatty liver disease and risk of fatal and non-fatal cardiovascular events: an updated systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2021;6:903–913. - PubMed
-
- Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J Hepatol. 2023;79:1542–1556. - PubMed
-
- Hagström H, Vessby J, Ekstedt M, Shang Y. 99% of patients with NAFLD meet MASLD criteria and natural history is therefore identical. J Hepatol. 2024;80:e76–e77. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 24K11078/Ministry of Education, Culture, Sports, Science, and Technology
- JP24fk0210114/Japan Agency for Medical Research and Development
- 24fk0210150h0001/Japan Agency for Medical Research and Development
- 21A2009/National Center for Global Health and Medicine
- 23A2014/National Center for Global Health and Medicine
LinkOut - more resources
Full Text Sources

