Impact of Pharmacogenetics on Pharmacokinetics of First-Line Antituberculosis Drugs in the HIRIF Trial
- PMID: 40239986
- PMCID: PMC12349936
- DOI: 10.1093/infdis/jiaf195
Impact of Pharmacogenetics on Pharmacokinetics of First-Line Antituberculosis Drugs in the HIRIF Trial
Abstract
Background: Variability in the pharmacokinetics (PK) of first-line antituberculosis drugs (rifampicin [RIF], isoniazid [INH], and pyrazinamide [PZA]) is high and may be influenced by pharmacogenetic polymorphism. We performed a pharmacogenetic substudy in 90 participants with PK data from the HIRIF trial in Peru.
Methods: Relevant single-nucleotide polymorphisms (SNPs) in the NAT2, SLCO1B1, AADAC, and AOX1 loci were genotyped using real-time polymerase chain reaction (PCR).
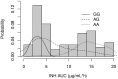
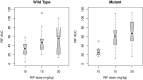
Results: The proportions of slow, intermediate, and fast acetylators predicted by a conventional 6-SNP NAT2 panel were 32.5%, 48.2%, and 19.2%, respectively. A single NAT2 tag SNP (rs1495741) agreed with the panel-predicted phenotype in 91% and was a better predictor of INH area under the curve (AUC). Accounting for discrepancies possibly caused by rare alleles not represented in the panel or that could be unequivocally resolved using observed AUC, sensitivity of the tag SNP was 97.7%. A previously described SNP in SLCO1B1 (rs4149032) was present at an allele frequency of 0.31 and appeared to influence RIF AUC and maximum concentration (Cmax) at a dose of 20 mg/kg, despite an extreme distribution of alleles across the randomized arms. The AADAC SNP (rs1803155) predominated in the study population and was not linked to RIF PK, although an effect could have been missed due to sample size and allele frequency. There was no association between PZA PK and a common SNP in AOX1 (rs55754655).
Conclusions: A tag SNP approach may offer simpler and cheaper prediction of INH PK. Further exploration of the impact of SLCO1B1 SNPs on RIF PK is required in this and other populations. Clinical Trials Registration. NCT01408914.
Keywords: NAT2; SLCO1B1; isoniazid; pharmacogenetics; pharmacokinetics; rifampicin; tuberculosis.
© The Author(s) 2025. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed. Rifampicin for the HIRIF study was donated by Sanofi-Aventis.
Figures

References
-
- Tiberi S, du Plessis N, Walzl G, et al. Tuberculosis: progress and advances in development of new drugs, treatment regimens, and host-directed therapies. Lancet Infect Dis 2018; 18:e183–98. - PubMed
-
- Hong BL, D'Cunha R, Li P, et al. A systematic review and meta-analysis of isoniazid pharmacokinetics in healthy volunteers and patients with tuberculosis. Clin Ther 2020; 42:e220–41. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

