Cellular and molecular mechanisms in the pathogenesis of pouchitis: more than just the microbiota
- PMID: 40240062
- PMCID: PMC12240151
- DOI: 10.1136/gutjnl-2024-334445
Cellular and molecular mechanisms in the pathogenesis of pouchitis: more than just the microbiota
Abstract
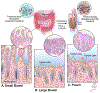
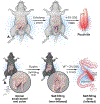
Pouchitis, defined as inflammation of the ileal pouch, is the most common complication following restorative proctocolectomy for refractory ulcerative colitis. Antibiotics remain the first line of therapy for pouchitis, but the majority of patients develop subsequent episodes and some are refractory to antibiotic therapy. This highlights the need for more effective treatment options and points to a more complex pathophysiology beyond the role of th pouch microbiome, similar to what is seen in inflammatory bowel disease. In this review, we outline the putative mechanisms of pouchitis, including genetic predisposition, microbiome alterations, dysfunction of the intestinal barrier and the immune system and review the available animal models of pouchitis. In addition, we introduce the concept of pouchitis as a possible transmural disease and discuss the potential role of non-immune cells, including stromal cells, in perpetuating inflammation and intestinal barrier dysfunction. We discuss future directions, implications for novel therapies and propose novel multicellular disease models that can better capture the complexity of pouchitis pathogenesis.
Keywords: GASTROINTESTINAL PHYSIOLOGY; ILEOANAL POUCH; POUCHITIS; ULCERATIVE COLITIS.
© Author(s) (or their employer(s)) 2025. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: MBBN, CF and AI: none; TQ: consultant for AbbVie, Celgene/Bristol Myers Squibb, Prometheus Biosciences and Janssen. SH: consultant to Takeda. FR: Advisory board or consulting: Adiso, Adnovate, Agomab, Allergan, AbbVie, Arena, Astra Zeneca, Bausch & Lomb, Boehringer-Ingelheim, Celgene/BMS, Celltrion, CDISC, Celsius, Cowen, 89Bio, Eugit, Ferring, Galapagos, Galmed, Genentech, Gilead, Gossamer, Granite, Guidepoint, Helmsley, Horizon Therapeutics, Image Analysis, Index Pharma, Landos, Jannsen, Koutif, Mestag, Metacrine, Mirum, Mobius, Mopac, Morphic, Myka Labs, Organovo, Origo, Palisade, Pfizer, Pliant, Prometheus Biosciences, Receptos, RedX, Roche, Samsung, Sanofi, Surmodics, Surrozen, Takeda, Techlab, Teva, Theravance, Thetis, Tr1x Bio, UCB, Ysios.
Figures

Similar articles
-
Is Ustekinumab Effective in Refractory Crohn's Disease of the Pouch and Chronic Pouchitis? A Systematic Review.Dig Dis Sci. 2022 Jun;67(6):1948-1955. doi: 10.1007/s10620-021-07002-5. Epub 2021 Jun 7. Dig Dis Sci. 2022. PMID: 34097166
-
Endoscopic Normalization and Transition of J-Pouch Phenotypes Over Time in Patients With Inflammatory Bowel Disease.Inflamm Bowel Dis. 2025 Jan 6;31(1):63-71. doi: 10.1093/ibd/izae106. Inflamm Bowel Dis. 2025. PMID: 38916136 Free PMC article.
-
Treatment and prevention of pouchitis after ileal pouch-anal anastomosis for chronic ulcerative colitis.Cochrane Database Syst Rev. 2010 Jun 16;(6):CD001176. doi: 10.1002/14651858.CD001176.pub2. Cochrane Database Syst Rev. 2010. Update in: Cochrane Database Syst Rev. 2015 Nov 23;(11):CD001176. doi: 10.1002/14651858.CD001176.pub3. PMID: 20556748 Updated.
-
Cyclosporine A for induction of remission in severe ulcerative colitis.Cochrane Database Syst Rev. 2005 Jan 25;(1):CD004277. doi: 10.1002/14651858.CD004277.pub2. Cochrane Database Syst Rev. 2005. PMID: 15674937
-
Prevalence of pouchitis in both ulcerative colitis and familial adenomatous polyposis: A systematic review and meta-analysis.Colorectal Dis. 2022 Jan;24(1):27-39. doi: 10.1111/codi.15995. Epub 2021 Dec 3. Colorectal Dis. 2022. PMID: 34800326
References
-
- Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology 2011;140:1785–94. - PubMed
-
- Parragi L, Fournier N, Zeitz J, Scharl M, Greuter T, Schreiner P, et al. Colectomy Rates in Ulcerative Colitis are Low and Decreasing: 10-year Follow-up Data From the Swiss IBD Cohort Study. J Crohns Colitis 2018;12:811–8. - PubMed
-
- Quinn KP, Raffals LE. An Update on the Medical Management of Inflammatory Pouch Complications. Am J Gastroenterol 2020;115:1439–50. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
