Immune-related adverse events among patients with early-stage triple-negative breast cancer treated with pembrolizumab plus chemotherapy: real-world data from the Neo-Real/GBECAM 0123 study
- PMID: 40240201
- PMCID: PMC12496205
- DOI: 10.1016/j.breast.2025.104473
Immune-related adverse events among patients with early-stage triple-negative breast cancer treated with pembrolizumab plus chemotherapy: real-world data from the Neo-Real/GBECAM 0123 study
Abstract
Background: Pembrolizumab combined with neoadjuvant chemotherapy is the standard of care for stage II-III triple-negative breast cancer (TNBC) based on the KEYNOTE-522 trial. However, 13 % of patients experienced immune-related adverse events (irAEs) of grade ≥3 in the trial. This study aims to describe patterns of irAEs in a real-world scenario during treatment with pembrolizumab for early-stage TNBC.
Methods: Patients treated with neoadjuvant pembrolizumab plus chemotherapy across ten Brazilian cancer centers were evaluated in the Neo-Real/GBECAM0123 study. This analysis focuses on irAE evaluation, including time to onset, management, and association between irAEs and pathological complete response (pCR).
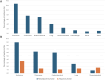
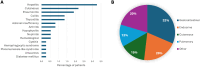
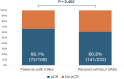
Results: A total of 368 patients were included. Overall, 31 % of patients (n = 114) presented with any grade irAEs. Most of irAEs (72.8 %) occurred during the neoadjuvant phase while 28.1 % happened during the adjuvant period. The most frequent irAEs were endocrine (12.8 % of the entire cohort), cutaneous (7.6 %) and gastrointestinal (7.1 %). A total of 50 patients (13.6 %) experienced grade ≥3 irAEs, predominantly gastrointestinal (32 %). 58 patients (56 %) needed corticosteroids. Immunotherapy rechallenge was possible in 53 % of the cases; permanent discontinuation of pembrolizumab was necessary for 16 %. No significant association was observed between irAEs and clinic-pathologic features nor pCR status.
Conclusions: In this real-world analysis, we observed a similar incidence of irAEs as reported in the KEYNOTE-522 trial. Most patients experienced resolution of their irAEs, but some required permanent discontinuation of pembrolizumab. Additionally, there were lasting dysfunctions, particularly endocrine, demanding lifelong support. Careful monitoring and management of these events are essential.
Keywords: Immune-related adverse events; Immunotherapy; Triple-negative breast cancer.
Copyright © 2025 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interest statement The Authors declare no Competing Non-Financial Interests but the following Competing Financial Interests: RCB: Speaker fees and/or honoraria for consulting or advisory functions: Daiichi-Sankyo, Nestle Health Science, Addium, Gilead, MSD, BMS, AstraZeneca, Ache, Pfizer. Financial support for educational programs and symposia: AstraZeneca, Daiichi-Sankyo, MSD, Lilly. Institutional Research grant: Novartis, AstraZeneca. JB: Speaker fees and/or honoraria for consulting or advisory functions: AstraZeneca, Daiichi-Sankyo, Lilly, Gilead, Pfizer, Novartis, MSD, Roche, Knight Pharmaceuticals. Financial support for educational programs and symposia: Roche, Daiichi-Sankyo. DDR: Speaker fees and/or honoraria for consulting or advisory functions: AstraZeneca, Daiichi-Sankyo, Lilly, Libbs, Pfizer, Novartis, Roche, GSK, Sanofi, Amgen, Zodiac Pharma. Financial support for educational programs and symposia: Roche. DAS: Speaker fees and/or honoraria for consulting or advisory functions: Daiichi-Sankyo. Financial support for educational programs and symposia: AstraZeneca. JAPA: Speaker fees and/or honoraria for consulting or advisory functions: Novartis, AstraZeneca, MSD, Lilly. DMG: Speaker fees and/or honoraria for consulting or advisory functions: Daiichi-Sankyo, Teva, Roche, AstraZeneca, Pfizer, Lilly, Novartis. Financial support for educational programs and symposia: AstraZeneca, Libbs, Roche. Research grant: Novartis. BMZ: AstraZeneca, Daiichi-Sankyo, Eli Lilly, Gilead, Pfizer, Novartis, MSD, Roche, Addium. AF: Speaker fees and/or honoraria for consulting or advisory functions: Daiichi-Sankyo, Novartis, Gilead, MSD, BMS, AstraZeneca, Pfizer. Financial support for educational programs and symposia: AstraZeneca, Daiichi-Sankyo, MSD, Novartis. CHA: Speaker fees and/or honoraria for consulting or advisory functions: Daiichi-Sankyo, Gilead AstraZeneca, Novartis, MSD. Financial support for educational programs and symposia: AstraZeneca, Daiichi-Sankyo, MSD, Lilly, Rcohe, Novartis, Gilead, Medscape. RC: Speaker fees and/or honoraria for consulting or advisory functions: AstraZeneca, Daichii-Sankyo, Eli Lilly, Gilead, MSD, and GSK. MMFM: Speaker fees and/or honoraria for consulting or advisory functions: AstraZeneca, Daiichi-Sankyo, Eli Lilly, Gilead, Adium, Novartis, MSD, and Roche. Financial support for educational programs and symposia: AstraZeneca, Daiichi-Sankyo, Gilead, Eli Lilly, Roche, MSD and Novartis. PMH: Speaker fees and/or honoraria for consulting or advisory functions: Daiichi-Sankyo. MPED: Speaker fees and/or honoraria for consulting or advisory functions: AstraZeneca. LT: Speaker fees and/or honoraria for consulting or advisory functions: Daiichi-Sankyo, MSD, AstraZeneca, Pfizer, Lilly, Novartis, Roche, Pfizer. Financial support for educational programs and symposia: AstraZeneca, Roche, Gilead, MSD. Institutional Research grant: Novartis. RBS: Speaker fees and/or honoraria for consulting or advisory functions: AstraZeneca, Daiichi-Sankyo, Eli Lilly, Gilead, Libbs, Pfizer, Novartis, MSD, and Roche. Financial support for educational programs and symposia: AstraZeneca, Daiichi-Sankyo, Gilead, Eli Lilly, and MSD. Institutional Research grant: AstraZeneca, Daiichi-Sankyo. MCG: Speaker fees and/or honoraria for consulting or advisory functions: GSK, Novartis, Knight Therapeutics, MSD. MOA, IGG, IMS, FCB, ACMC, MCT, FM, RPF, CLS, and ZSS declare no conflict of interest.
Figures


References
-
- Schmid P., Cortés J., Dent R., et al. Abstract LBO1-01: neoadjuvant pembrolizumab or placebo plus chemotherapy followed by adjuvant pembrolizumab or placebo for early-stage triple-negative breast cancer: updated event-free survival results from the phase 3 KEYNOTE-522 study. Cancer Res. 2024;84(9_Supplement) doi: 10.1158/1538-7445.sabcs23-lbo1-01. LBO1-01-LBO1-01. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

