Cystic Fibrosis-related neurodegenerative disease associated with tauopathy and cognitive decline in aged CF mice
- PMID: 40240239
- PMCID: PMC12403229
- DOI: 10.1016/j.jcf.2025.04.003
Cystic Fibrosis-related neurodegenerative disease associated with tauopathy and cognitive decline in aged CF mice
Abstract
Background: Highly effective modulator therapies (HEMT) are increasing the lifespan for many people with cystic fibrosis (pwCF), making it necessary to identify and understand CF specific age-related consequences. In this study, we examine the impact of aging on cognitive function and age-related brain pathology in a CF mouse model focusing on phospho-Tau (pTau) pathology.
Methods: Cognitive function was measured by novel object recognition and spontaneous alternation behavior tests. Hippocampal neuronal function was assessed by measuring long-term potentiation (LTP) electrophysiology, the synaptic correlate of learning and memory. Tau pathology was assessed by immunohistochemical analyses and western blot assessment of pTau levels in CF mouse brain, as well as human nasal epithelial cells isolated from pwCF.
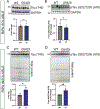
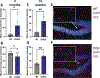
Results: Cognitive function declined progressively with age in Cftr (G542X/G542X) (G542X) mice, a model of CF, compared to wild-type (WT) littermate controls. LTP was also deficient in older G542X mice. Increased pTau was observed by staining and western blot analysis in the hippocampus of aged CF mice. Secondary impacts of tauopathy, including increased microglial uptake of cholesterol and reduced neuronal density were also observed. Lastly, human nasal epithelial cells from pwCF were found to display elevated pTau levels compared to non-CF controls.
Conclusions: Aging CF mice develop tauopathy, cognitive decline, LTP impairment, microglial activation, and neurodegeneration that is not experienced by age-matched WT littermates, a condition herein termed cystic fibrosis-related neurodegeneration (CFND). These findings suggest that pwCF may be at risk for tauopathy-related neurodegeneration and cognitive impairment with aging.
Keywords: Cognition; Cystic fibrosis; Dementia; Tauopathy.
Copyright © 2025. Published by Elsevier B.V.
Conflict of interest statement
Declaration of competing interest No authors have any conflicting interests with this work.
Figures



References
-
- Drumm ML, Collins FS. Molecular biology of cystic fibrosis. Mol. Genet. Med. 1993;3:33–68. - PubMed
-
- Chadwick HK, Abbott J, Hurley MA, Dye L, Lawton CL, Mansfield MW, et al. Cystic fibrosis-related diabetes (CFRD) and cognitive function in adults with cystic fibrosis. J. Cyst. Fibros. 2022;21(3):519–28. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

