C12ORF49 inhibits ferroptosis in hepatocellular carcinoma cells via reprogramming SREBP1/SCD1-mediated lipid metabolism
- PMID: 40240331
- PMCID: PMC12003882
- DOI: 10.1038/s41420-025-02480-2
C12ORF49 inhibits ferroptosis in hepatocellular carcinoma cells via reprogramming SREBP1/SCD1-mediated lipid metabolism
Abstract
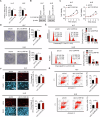
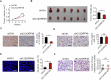
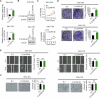
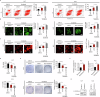
Altered lipid metabolism is an emerging hallmark of cancer, which is involved in various aspects of the cancer phenotypes. C12ORF49 has recently been identified as a pivotal regulator of sterol regulatory element binding proteins (SREBPs), a family of transcriptional factors that govern lipid biosynthesis. Nevertheless, the function of C12ORF49 in human cancers has not been studied. Here, we show that C12ORF49 levels are higher in HCC tissue than in nearby non-cancerous liver tissue. Additionally, increased C12ORF49 expression is linked to poorer survival outcomes in HCC patients. Functional experiments uncovered that knockdown of C12ORF49 inhibited HCC cell survival and tumor growth by inducing ferroptosis, whereas the opposites were observed upon C12ORF49 overexpression. Mechanistically, C12ORF49 promotes SREBP1/SCD-regulated production of monounsaturated fatty acids, which inhibits ferroptosis in HCC cells. Furthermore, silencing C12ORF49 combined with Sorafenib treatment showed a synergistic effect in inducing HCC cell death. Together, our findings suggest a critical role of C12ORF49 in the evasion of ferroptosis in HCC cells, highlighting the potential of targeting C12ORF49 as a therapeutic strategy to enhance the efficacy of Sorafenib treatment in HCC.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests Ethics approval: This study has been approved by the Ethics Committee of Xijing Hospital and all methods were performed in accordance with the relevant guidelines and regulations.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources

