Chromosome end protection by RAP1-mediated inhibition of DNA-PK
- PMID: 40240611
- PMCID: PMC12221994
- DOI: 10.1038/s41586-025-08896-1
Chromosome end protection by RAP1-mediated inhibition of DNA-PK
Erratum in
-
Author Correction: Chromosome end protection by RAP1-mediated inhibition of DNA-PK.Nature. 2025 Jun;642(8067):E15. doi: 10.1038/s41586-025-09121-9. Nature. 2025. PMID: 40410578 Free PMC article. No abstract available.
Abstract
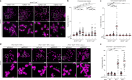
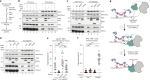
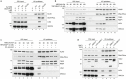
During classical non-homologous end joining (cNHEJ), DNA-dependent protein kinase (DNA-PK) encapsulates free DNA ends, forming a recruitment platform for downstream end-joining factors including ligase 4 (LIG4)1. DNA-PK can also bind telomeres and regulate their resection2-4, but does not initiate cNHEJ at this position. How the end-joining process is regulated in this context-specific manner is currently unclear. Here we show that the shelterin components TRF2 and RAP1 form a complex with DNA-PK that directly represses its end-joining function at telomeres. Biochemical experiments and cryo-electron microscopy reveal that when bound to TRF2, RAP1 establishes a network of interactions with KU and DNA that prevents DNA-PK from recruiting LIG4. In mouse and human cells, RAP1 is redundant with the Apollo nuclease in repressing cNHEJ at chromosome ends, demonstrating that the inhibition of DNA-PK prevents telomere fusions in parallel with overhang-dependent mechanisms. Our experiments show that the end-joining function of DNA-PK is directly and specifically repressed at telomeres, establishing a molecular mechanism for how individual linear chromosomes are maintained in mammalian cells.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures











References
-
- de Lange, T. Shelterin-mediated telomere protection. Annu. Rev. Genet.52, 223–247 (2018). - PubMed
-
- d’Adda di Fagagna, F. et al. Effects of DNA nonhomologous end-joining factors on telomere length and chromosomal stability in mammalian cells. Curr. Biol.11, 1192–1196 (2001). - PubMed
-
- Denchi, E. L. & de Lange, T. Protection of telomeres through independent control of ATM and ATR by TRF2 and POT1. Nature448, 1068–1071 (2007). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

