Impaired cardiac branched-chain amino acid metabolism in a novel model of diabetic cardiomyopathy
- PMID: 40240904
- PMCID: PMC12004671
- DOI: 10.1186/s12933-025-02725-5
Impaired cardiac branched-chain amino acid metabolism in a novel model of diabetic cardiomyopathy
Abstract
Background: Systemic insulin resistance plays an important role in the pathogenesis of type 2 diabetes and its complications. Although impaired branched-chain amino acid (BCAA) metabolism has been reported to be involved in the development of diabetes, the relationship between cardiac BCAA metabolism and the pathogenesis of diabetic cardiomyopathy (DbCM) remains unclear.
Objectives: The aim of this study was to investigate BCAA metabolism in insulin-resistant hearts by using a novel mouse model of DbCM.
Methods: The cardiac phenotypes of adipocyte-specific 3'-phosphoinositide-dependent kinase 1 (PDK1)-deficient (A-PDK1KO) mice were assessed by histological analysis and echocardiography. The metabolic characteristics and cardiac gene expression were determined by mass spectrometry or RNA sequencing, respectively. Cardiac protein expression was evaluated by Western blot analysis.
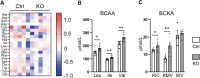
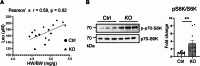
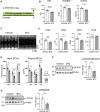
Results: A-PDK1KO mouse hearts exhibited hypertrophy with prominent insulin resistance, consistent with cardiac phenotypes and metabolic disturbances previously reported as DbCM characteristics. RNA sequencing revealed the activation of BCAA uptake in diabetic hearts. In addition, the key enzymes involved in cardiac BCAA catabolism were downregulated at the protein level in A-PDK1KO mice, leading to the accumulation of BCAAs in the heart. Mechanistically, the accumulation of the BCAA leucine caused cardiac hypertrophy via the activation of mammalian target of rapamycin complex 1 (mTORC1).
Conclusions: A-PDK1KO mice closely mimic the cardiac phenotypes and metabolic alterations observed in human DbCM and exhibit impaired BCAA metabolism in the heart. This model may contribute to a better understanding of DbCM pathophysiology and to the development of novel therapies for this disease.
Keywords: Branched-chain amino acid; Cardiac metabolism; Diabetes mellitus; Diabetic cardiomyopathy; Heart failure.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All animal studies were conducted in accordance with institutional guidelines and the Guide for the Care and Use of Laboratory Animals after receiving the approval of Kobe University. Consent for publication: Not applicable. Competing interests: The Division of Evidence-based Laboratory Medicine, Kobe University Graduate School of Medicine, was established by an endowment fund from Sysmex Corporation, Japan.
Figures





References
-
- Tinajero MG, Malik VS. An update on the epidemiology of type 2 diabetes: a global perspective. Endocrinol Metab Clin North Am. 2021;50(3):337–55. - PubMed
-
- Kim AH, Jang JE, Han J. Current status on the therapeutic strategies for heart failure and diabetic cardiomyopathy. Biomed Pharmacother. 2022;145:112463. - PubMed
-
- Nichols GA, Hillier TA, Erbey JR, Brown JB. Congestive heart failure in type 2 diabetes: prevalence, incidence, and risk factors. Diabetes Care. 2001;24(9):1614–9. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

