Yersinia pestis Actively Inhibits the Production of Extracellular Vesicles by Human Neutrophils
- PMID: 40240908
- PMCID: PMC12003101
- DOI: 10.1002/jev2.70074
Yersinia pestis Actively Inhibits the Production of Extracellular Vesicles by Human Neutrophils
Abstract
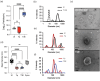
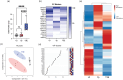
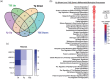
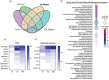
Yersinia pestis is the etiologic agent of the plague. A hallmark of plague is subversion of the host immune response by disrupting host signalling pathways required for inflammation. This non-inflammatory environment permits bacterial colonization and has been shown to be essential for disease manifestation. Previous work has shown that Y. pestis inhibits phagocytosis and degranulation by neutrophils. Manipulation of these key vesicular trafficking pathways suggests that Y. pestis influences extracellular vesicle (EV) secretion, cargo selection, trafficking and/or maturation. Our goals were to define the EV population produced by neutrophils in response to Y. pestis and determine how these vesicles might influence inflammation. Towards these goals, EVs were isolated from human neutrophils infected with Y. pestis or a mutant lacking bacterial effector proteins known to manipulate host cell signalling. Mass spectrometry data revealed that cargoes packaged in EVs isolated from mutant infected cells were enriched with antimicrobial and cytotoxic proteins, contents which differed from uninfected and Y. pestis infected cells. Further, EVs produced in response to Y. pestis lacked inflammatory properties observed in those isolated from neutrophils responding to the mutant. Together, these data demonstrate that Y. pestis actively inhibits the production of antimicrobial EVs produced by neutrophils, likely contributing to immune evasion.
Keywords: Yersinia pestis; Yop effectors; human neutrophils (hPMNs); plague; type 3 secretion system (T3SS).
© 2025 The Author(s). Journal of Extracellular Vesicles published by Wiley Periodicals, LLC on behalf of the International Society for Extracellular Vesicles.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Update of
-
Yersinia pestis actively inhibits the production of extracellular vesicles by human neutrophils.bioRxiv [Preprint]. 2024 Dec 21:2024.12.20.629761. doi: 10.1101/2024.12.20.629761. bioRxiv. 2024. Update in: J Extracell Vesicles. 2025 Apr;14(4):e70074. doi: 10.1002/jev2.70074. PMID: 39763979 Free PMC article. Updated. Preprint.
References
-
- Andor, A. , Trulzsch K., Essler M., et al. 2001. “YopE of Yersinia, a GAP for Rho GTPases, Selectively Modulates Rac‐dependent Actin Structures in Endothelial Cells.” Cellular Microbiology 3, no. 5: 301–310. - PubMed
MeSH terms
Grants and funding
- P20GM103436/GM/NIGMS NIH HHS/United States
- P20GM113226/GM/NIGMS NIH HHS/United States
- P20 GM103436/GM/NIGMS NIH HHS/United States
- R01AI148241/National Institute of Allergy and Infectious Diseases
- F31AI178999/National Institute of Allergy and Infectious Diseases
- R21 AI169423/AI/NIAID NIH HHS/United States
- R01 AI178106/AI/NIAID NIH HHS/United States
- Jewish Heritage for Excellence Fund
- F31 AI178999/AI/NIAID NIH HHS/United States
- R01AI178106/National Institute of Allergy and Infectious Diseases
- P20 GM113226/GM/NIGMS NIH HHS/United States
- R01 AI148241/AI/NIAID NIH HHS/United States
- University of Louisville Proteomics Technology Center
- R21AI169423/National Institute of Allergy and Infectious Diseases
LinkOut - more resources
Full Text Sources
Medical

