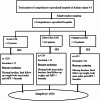
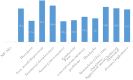
Patients reported neuropsychiatric adverse events and associated factors among PLHIV patients receiving DTG-based regimen antiretroviral therapy real-life clinical practice in Ethiopia: multi center crossetional study
- PMID: 40241041
- PMCID: PMC12004852
- DOI: 10.1186/s12888-025-06820-5
Patients reported neuropsychiatric adverse events and associated factors among PLHIV patients receiving DTG-based regimen antiretroviral therapy real-life clinical practice in Ethiopia: multi center crossetional study
Abstract
Background: Dolutegravir-based antiretroviral therapy has established itself as a key component for treating individuals with HIV. In reality, clinical settings in resource-poor areas like Ethiopia often face difficulties when using dolutegravir-based treatment due to neuropsychiatric side effects, which can not only reduce the therapy's effectiveness but also lead to non-adherence, switching, diminished therapeutic outcomes and discontinuation. This research aimed to determine the prevalence and factors related to neuropsychiatric side effects among people living with HIV who recently began receiving Dolutegravir-based Antiretroviral Therapy in real-life clinical settings in Ethiopia.
Method: A multicenter cross-sectional study was conducted from December 1, 2023, to August 30, 2023, peoples with HIV who were initiating a Dolutegravir-based regimen for the first time. Data collection was conducted using a structured questionnaire designed to gather sociodemographic, clinical characteristics, and neuropsychiatric adverse effects from patient interviews and medical records. Bivariate and multivariate analyses were employed to identify variables significantly associated with neuropsychiatric adverse events, with P-value of ≤ 0.05.
Results: A total of 620 participants were included in the analysis, revealing a 28.3% prevalence of neuropsychiatric adverse events. The most commonly reported events were depression, insomnia, and aggressive mood. Being female [AOR = 1.72], age between 47 and 54 years [AOR = 3.8] and 55-75 years [AOR = 1.27], unemployment status [AOR = 1.35], lack of physical activity [AOR = 3.6], and minimal of physical activity [AOR = 2.4], duration since DTG-based regimen initiation, 6-12 months[AOR = 3.35], and 13-24 months [AOR = 2.67], WHO clinical stage II [AOR = 3.65], III and IV [AOR = 4.21], Detectable viral load level at the start therapy [AOR = 1.24 (1.97.6-2.05)] were significantly associated with neuropsychiatric adverse events.
Conclusion: Being female, aged 47-75 years, unemployed, engaged in minimal or no physical activity, having a treatment duration of 6-24 months, advanced disease stage (WHO stage II-IV) and had a detectable viral load at the start of therapy. Monitoring these groups for neuropsychiatric adverse events is essential to facilitate timely interventions and improve patient outcomes.
Keywords: Antiretroviral therapy; Ethiopia; Neuropsychiatric adverse events; PLHIV dolutegravir based regimen.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The University of Gondar College of Medicine and Health Sciences School of Pharmacy Ethical approval Review Committee October 22, 2023, SOP (088/2023) granted ethics approval. All respondents provided informed consent for participation in the survey. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
Similar articles
-
Neuropsychiatric outcomes before and after switching to dolutegravir-based therapy in an acute HIV cohort.AIDS Res Ther. 2020 Jan 7;17(1):1. doi: 10.1186/s12981-019-0257-8. AIDS Res Ther. 2020. PMID: 31907064 Free PMC article.
-
Prevalence and factors associated with adverse drug events among patients on dolutegravir-based regimen at the Immune Suppression Syndrome Clinic of Mbarara Regional Referral Hospital, Uganda: a mixed design study.AIDS Res Ther. 2022 Apr 2;19(1):18. doi: 10.1186/s12981-022-00442-7. AIDS Res Ther. 2022. PMID: 35366917 Free PMC article.
-
Unveiling the prevalence of anaemia and its predictors among adults on highly active antiretroviral therapy in the dolutegravir era: a retrospective cross-sectional study.BMJ Open. 2024 Sep 5;14(9):e086480. doi: 10.1136/bmjopen-2024-086480. BMJ Open. 2024. PMID: 39242159 Free PMC article.
-
Doing More With Less: Review of Dolutegravir-Lamivudine, a Novel Single-Tablet Regimen for Antiretroviral-Naïve Adults With HIV-1 Infection.Ann Pharmacother. 2020 Dec;54(12):1252-1259. doi: 10.1177/1060028020933772. Epub 2020 Jun 9. Ann Pharmacother. 2020. PMID: 32517480 Review.
-
Prevalence of Emergent Dolutegravir Resistance Mutations in People Living with HIV: A Rapid Scoping Review.Viruses. 2024 Mar 4;16(3):399. doi: 10.3390/v16030399. Viruses. 2024. PMID: 38543764 Free PMC article.
Cited by
-
Biomedical Interventions for HIV Prevention and Control: Beyond Vaccination.Viruses. 2025 May 26;17(6):756. doi: 10.3390/v17060756. Viruses. 2025. PMID: 40573348 Free PMC article. Review.
References
-
- Vitoria M, Hill A, Ford N, Doherty M, Clayden P, Venter F, et al. The transition to dolutegravir and other new antiretrovirals in low-income and middle-income countries: what are the issues? Aids. 2018;32(12):1551–61. - PubMed
-
- Povar-Echeverría M, Comet-Bernad M, Gasso-Sánchez A, Ger-Buil A, Navarro-Aznarez H, Martínez-Álvarez R, et al. Neuropsychiatric adverse effects of dolutegravir in real-life clinical practice. Enfermedades Infecciosas Y Microbiol Clin (English ed). 2021;39(2):78–82. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical