A central role for CCR2 in monocyte recruitment and blood-brain barrier disruption during Usutu virus encephalitis
- PMID: 40241134
- PMCID: PMC12004732
- DOI: 10.1186/s12974-025-03435-1
A central role for CCR2 in monocyte recruitment and blood-brain barrier disruption during Usutu virus encephalitis
Abstract
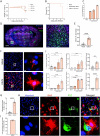
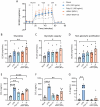
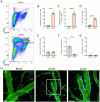
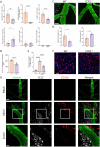
Usutu virus (USUV) is an emerging neurotropic flavivirus capable of causing encephalitis in humans. Here, our main goal was to characterize the innate immune response in the brain during USUV encephalitis and to identify strategies to control disease severity. Using an immunocompetent mouse model of USUV encephalitis, we showed that microglia activation, blood-brain barrier (BBB) disruption and inflammatory monocyte recruitment are hallmarks of disease 6 days post infection. Activated microglia were in close association to USUV-infected cells, concomitantly with elevated levels of IL-6, IFN-γ, CCL2, CCL5, CXCL10 and CXCL1 in the brain. Monocyte recruitment was CCR2-dependent and driven by IFN-γ and CCL2 production beneath the brain vasculature. Moreover, CCR2 deficiency inhibited microglia activation and BBB disruption, showing the central role of CCR2 in USUV encephalitis. Accordingly, treatment with dexamethasone prevented pro-inflammatory mediator production and reduced leukocyte recruitment significantly, restraining encephalitis severity. Concluding, USUV encephalitis is driven by CCR2-mediated monocyte recruitment and BBB disruption, and blocked therapeutically by glucocorticoids.
Keywords: Blood–brain barrier; CCR2; Encephalitis; Glucocorticoid; Microglia; Monocyte; Usutu virus.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Experimental procedures were approved and performed following the guidelines of the Animal Ethics Committee from KU Leuven (registry number: P084/2022). Consent for publication: All authors consent the publication. Competing interests: The authors declare no competing interests.
Figures


References
-
- Sips GJ, Wilschut J, Smit JM. Neuroinvasive flavivirus infections. Rev Med Virol. 2012;22(2):69–87. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

