An Update: Enzymatic Synthesis for Industrial Applications
- PMID: 40241335
- PMCID: PMC12207387
- DOI: 10.1002/anie.202505976
An Update: Enzymatic Synthesis for Industrial Applications
Abstract
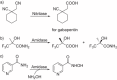
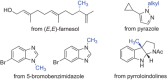
Supported by rapid technological advancements, biocatalytic applications have matured into sustainable, scalable, and cost-competitive alternatives to established chemical catalysis. This review presents the most recent examples of enzyme-based solutions for the manufacturing of molecules with extended carbon-carbon frameworks and multiple stereogenic centers at commercial scale, including peptide building blocks, (rare) sugars, synthetic (oligo)nucleotides, and terpenoids, such as (-)-Ambrox®. Novel enzyme classes are highlighted along with their potential applications-the synthesis of DNA/RNA, the depolymerization of synthetic plastics, or fully enzymatic protection/deprotection schemes-pointing toward the diversification and broader industrial utilization of biocatalysis-based processes.
Keywords: Biocatalysis; Enzyme catalysis; Industrial catalysis; Organic synthesis; Stereoselectivity.
© 2025 The Author(s). Angewandte Chemie International Edition published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures












Similar articles
-
Bioinspired Chiral Peptide-Phosphonium Salt Catalysis: From Enzymes to Cationic Small-Molecule Enzyme Mimics.Acc Chem Res. 2025 Jul 1;58(13):2088-2109. doi: 10.1021/acs.accounts.5c00257. Epub 2025 Jun 13. Acc Chem Res. 2025. PMID: 40513008
-
Fluorinated Radicals in Divergent Synthesis via Photoredox Catalysis.Acc Chem Res. 2025 Jul 1;58(13):2046-2060. doi: 10.1021/acs.accounts.5c00239. Epub 2025 Jun 11. Acc Chem Res. 2025. PMID: 40499907 Free PMC article.
-
Biocatalysis in Transforming Biofuel Technologies.Front Biosci (Elite Ed). 2025 Jun 19;17(2):37729. doi: 10.31083/FBE37729. Front Biosci (Elite Ed). 2025. PMID: 40613142 Review.
-
Commercial utilization of bacteriocins: tackling challenges and exploring their potential as alternatives to antibiotics.Future Microbiol. 2025 Jul;20(10):681-693. doi: 10.1080/17460913.2025.2520693. Epub 2025 Jun 19. Future Microbiol. 2025. PMID: 40536428 Review.
-
Sexual Harassment and Prevention Training.2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 36508513 Free Books & Documents.
Cited by
-
Integrated Chemoenzymatic Synthesis of the mRNA Vaccine Building Block N1-Methylpseudouridine Triphosphate.Angew Chem Int Ed Engl. 2025 Aug 18;64(34):e202506330. doi: 10.1002/anie.202506330. Epub 2025 Jul 4. Angew Chem Int Ed Engl. 2025. PMID: 40424092 Free PMC article.
References
-
- Bell E. L., Finnigan W., France S. P., Green A. P., Hayes M. A., Hepworth L. J., Lovelock S. L., Niikura H., Osuna S., Romero E., Ryan K. S., Turner N. J., Flitsch S. L., Nat. Rev. Methods Primer 2021, 1, 46.
-
- Lovelock S. L., Crawshaw R., Basler S., Levy C., Baker D., Hilvert D., Green A. P., Nature 2022, 606, 49–58. - PubMed
-
- Hanefeld U., Hollmann F., Paul C. E., Chem. Soc. Rev. 2022, 51, 594–627. - PubMed
-
- Connell A. O., Barry A., Burke A. J., Hutton A. E., Bell E. L., Green A. P., O'Reilly E., Chem. Soc. Rev. 2024, 53, 2828–2850. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

