Paeoniflorin Protects Retinal Pigment Epithelial Cells from High Glucose-Induced Oxidative Damage by Activating Nrf2-Mediated HO-1 Signaling
- PMID: 40241343
- PMCID: PMC12059356
- DOI: 10.4062/biomolther.2025.025
Paeoniflorin Protects Retinal Pigment Epithelial Cells from High Glucose-Induced Oxidative Damage by Activating Nrf2-Mediated HO-1 Signaling
Abstract
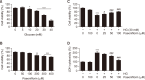
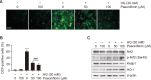
Oxidative stress due to hyperglycemia damages the functions of retinal pigment epithelial (RPE) cells and is a major risk factor for diabetic retinopathy (DR). Paeoniflorin is a monoterpenoid glycoside found in the roots of Paeonia lactiflora Pall and has been reported to have a variety of health benefits. However, the mechanisms underlying its therapeutic effects on high glucose (HG)-induced oxidative damage in RPE cells are not fully understood. In this study, we investigated the protective effect of paeoniflorin against HG-induced oxidative damage in cultured human RPE ARPE-19 cells, an in vitro model of hyperglycemia. Pretreatment with paeoniflorin markedly reduced HG-induced cytotoxicity and DNA damage. Paeoniflorin inhibited HG-induced apoptosis by suppressing activation of the caspase cascade, and this suppression was associated with the blockade of cytochrome c release to cytoplasm by maintaining mitochondrial membrane stability. In addition, paeoniflorin suppressed the HG-induced production of reactive oxygen species (ROS), increased the phosphorylation of nuclear factor erythroid 2-related factor 2 (Nrf2), a key redox regulator, and the expression of its downstream factor heme oxygenase-1 (HO-1). On the other hand, zinc protoporphyrin (ZnPP), an inhibitor of HO-1, abolished the protective effect of paeoniflorin against ROS production in HG-treated cells. Furthermore, ZnPP reversed the protective effects of paeoniflorin against HG-induced cellular damage and induced mitochondrial damage, DNA injury, and apoptosis in paeoniflorin-treated cells. These results suggest that paeoniflorin protects RPE cells from HG-mediated oxidative stress-induced cytotoxicity by activating Nrf2/HO-1 signaling and highlight the potential therapeutic use of paeoniflorin to improve the symptoms of DR.
Keywords: High glucose; Nrf2/HO-1; Oxidative stress; Paeoniflorin; Retinal pigment epithelial cells.
Conflict of interest statement
The authors have no conflicts of interest relevant to this study to disclose.
Figures


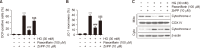


Similar articles
-
Activation of Nrf2/HO-1 antioxidant signaling correlates with the preventive effect of loganin on oxidative injury in ARPE-19 human retinal pigment epithelial cells.Genes Genomics. 2023 Mar;45(3):271-284. doi: 10.1007/s13258-022-01302-4. Epub 2022 Aug 26. Genes Genomics. 2023. PMID: 36018494
-
Fisetin Attenuated Oxidative Stress-Induced Cellular Damage in ARPE-19 Human Retinal Pigment Epithelial Cells Through Nrf2-Mediated Activation of Heme Oxygenase-1.Front Pharmacol. 2022 Jun 16;13:927898. doi: 10.3389/fphar.2022.927898. eCollection 2022. Front Pharmacol. 2022. PMID: 35784747 Free PMC article.
-
Albiflorin, a Monoterpene Glycoside, Protects Myoblasts against Hydrogen Peroxide-Induced Apoptosis by Activating the Nrf2/HO-1 Axis.Biomol Ther (Seoul). 2025 Jul 1;33(4):716-727. doi: 10.4062/biomolther.2025.020. Epub 2025 Jun 10. Biomol Ther (Seoul). 2025. PMID: 40492376 Free PMC article.
-
Aloperine protects human retinal pigment epithelial cells against hydrogen peroxide-induced oxidative stress and apoptosis through activation of Nrf2/HO-1 pathway.J Recept Signal Transduct Res. 2022 Feb;42(1):88-94. doi: 10.1080/10799893.2020.1850787. Epub 2020 Nov 30. J Recept Signal Transduct Res. 2022. PMID: 33256538
-
3H-1,2-dithiole-3-thione protects retinal pigment epithelium cells against Ultra-violet radiation via activation of Akt-mTORC1-dependent Nrf2-HO-1 signaling.Sci Rep. 2016 May 6;6:25525. doi: 10.1038/srep25525. Sci Rep. 2016. PMID: 27151674 Free PMC article.
References
-
- Bhat A. A., Moglad E., Goyal A., Afzal M., Thapa R., Almalki W. H., Kazmi I., Alzarea S. I., Ali H., Gaur A., Singh T. G., Singh S. K., Dua K., Gupta G. Nrf2 pathways in neuroprotection: alleviating mitochondrial dysfunction and cognitive impairment in aging. Life Sci. 2024;357:123056. doi: 10.1016/j.lfs.2024.123056. - DOI - PubMed
-
- Cano M., Datta S., Wang L., Liu T., Flores-Bellver M., Sachdeva M., Sinha D., Handa J. T. Nrf2 deficiency decreases NADPH from impaired IDH shuttle and pentose phosphate pathway in retinal pigmented epithelial cells to magnify oxidative stress-induced mitochondrial dysfunction. Aging Cell. 2021;20:e13444. doi: 10.1111/acel.13444. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

