Fibronectin-dependent integrin signaling drives EphA2 expression in vascular smooth muscle cells
- PMID: 40241381
- PMCID: PMC12482797
- DOI: 10.1152/ajpcell.01021.2024
Fibronectin-dependent integrin signaling drives EphA2 expression in vascular smooth muscle cells
Abstract
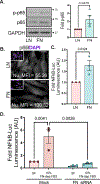
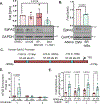
Vascular smooth muscle cells undergo a phenotypic shift to a "synthetic" phenotype during atherosclerosis characterized by downregulation of contractile markers and augmented proliferation, migration, and extracellular matrix deposition. While absent in contractile smooth muscle cells, the receptor tyrosine kinase EphA2 shows enhanced expression in synthetic vascular smooth muscle in vitro and in atherosclerotic plaques in vivo. EphA2 deletion in atheroprone ApoE knockout mice reduces plaque size, fibrous tissue, and smooth muscle content. However, the mechanisms regulating smooth muscle EphA2 expression remain unknown. Although serum strongly induces EphA2 expression, individual growth factors and insulin all failed to stimulate EphA2 expression in smooth muscle cells. In contrast, adhesion to fibronectin stimulated the expression of EphA2, while blunting serum-induced fibronectin deposition attenuated EphA2 expression, suggesting a critical role for fibronectin signaling. Fibronectin binds to a subset of extracellular matrix-binding integrins, and blocking fibronectin-integrin interactions or inhibiting specific fibronectin-binding integrins both attenuated EphA2 expression. Furthermore, pharmacological inhibition of fibronectin-binding integrins significantly reduced EphA2 expression in atherosclerotic plaques. RNA sequencing analysis of fibronectin-associated gene expression pointed to NF-κB as a likely transcription factor mediating fibronectin-responsive genes. Adhesion to fibronectin enhanced NF-κB activation in smooth muscle cells and inhibiting NF-κB blunted EphA2 expression associated with fibronectin. In addition, chromatin immunoprecipitation showed that NF-κB directly interacts with the EphA2 promoter, and mutating this site blunts fibronectin-dependent EphA2 promoter activity. Together these data identify a novel role for fibronectin-dependent integrin signaling in the induction of smooth muscle EphA2 expression.NEW & NOTEWORTHY Here, we demonstrate a novel interplay between cell-cell and cell-matrix adhesions, showing that fibronectin-dependent integrin signaling promotes NF-κB activation and interaction with the EphA2 promoter to drive smooth muscle EphA2 expression, whereas integrin inhibition attenuates EphA2 expression in atherosclerotic plaques in vivo. Although this relationship has clear implications on smooth muscle fibroproliferative remodeling in atherosclerosis, the matrix-specific regulation of EphA2 expression may impact a variety of pathological conditions.
Keywords: EphA2; NF-κB; fibronectin; integrins; vascular smooth muscle.
Conflict of interest statement
DISCLOSURES:
The authors have no conflicts to disclose.
Figures




References
-
- de Ruiter MC, Poelmann RE, van Iperen L, and Gittenberger-de Groot AC. The early development of the tunica media in the vascular system of rat embryos. Anat Embryol (Berl) 181: 341–349, 1990. - PubMed
-
- Jones BA, Aly HM, Forsyth EA, and Sidawy AN. Phenotypic characterization of human smooth muscle cells derived from atherosclerotic tibial and peroneal arteries. J Vasc Surg 24: 883–891, 1996. - PubMed
-
- Stary HC, Chandler AB, Dinsmore RE, Fuster V, Glagov S, Insull W Jr., Rosenfeld ME, Schwartz CJ, Wagner WD, and Wissler RW. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation 92: 1355–1374, 1995. - PubMed
MeSH terms
Substances
Grants and funding
- R00 HL150233/HL/NHLBI NIH HHS/United States
- HL139755/HHS | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- K99 HL150233/HL/NHLBI NIH HHS/United States
- Center for Cardiovascular Diseases and Sciences
- Malcolm Feist
- R00 HL145131/HL/NHLBI NIH HHS/United States
- HL167758/HHS | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- R01 HL172970/HL/NHLBI NIH HHS/United States
- R01 DK136685/DK/NIDDK NIH HHS/United States
- HL172970/HHS | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- HL145753-03S1/HHS | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- CA226285/HHS | NIH | National Cancer Institute (NCI)
- DK131859/HHS | NIH | National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)
- HL141155/HHS | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- R01 CA226285/CA/NCI NIH HHS/United States
- R01 DK134011/DK/NIDDK NIH HHS/United States
- HL145753-01S1/HHS | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- LSU | LSUS | LSU Health Shreveport (Louisiana Health Shreveport, Louisiana State University Shreveport)
- Carrol Feist
- R01 HL158546/HL/NHLBI NIH HHS/United States
- 17PRE33440111/American Heart Association (AHA)
- R01 HL145753/HL/NHLBI NIH HHS/United States
- R01 HL139755/HL/NHLBI NIH HHS/United States
- DK136685/HHS | NIH | National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)
- R01 HL167758/HL/NHLBI NIH HHS/United States
- 18POST34080495/American Heart Association (AHA)
- R01 HL141155/HL/NHLBI NIH HHS/United States
- HL145131/HHS | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- HL158546/HHS | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- 20POST35220022/American Heart Association (AHA)
- HL17397/HHS | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- R01 HL173972/HL/NHLBI NIH HHS/United States
- DK134011/HHS | NIH | National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)
- HL145753/HHS | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- HL150233/HHS | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- F31 DK131859/DK/NIDDK NIH HHS/United States
- R01 HL133497/HL/NHLBI NIH HHS/United States
- HL133497/HHS | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- 13GRNT17050093/American Heart Association (AHA)
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

