Efficacy of Second-Line Treatments After Atezolizumab and Bevacizumab in Advanced Hepatocellular Carcinoma and Related Prognostic Factors: A Multicenter Study by the Turkish Oncology Group (TOG)
- PMID: 40241388
- PMCID: PMC12070425
- DOI: 10.5152/tjg.2025.24784
Efficacy of Second-Line Treatments After Atezolizumab and Bevacizumab in Advanced Hepatocellular Carcinoma and Related Prognostic Factors: A Multicenter Study by the Turkish Oncology Group (TOG)
Abstract
Background/aims: The treatment of hepatocellular carcinoma (HCC), which accounts for 90% of all liver cancers, is highly varied. The use of second-line treatments following progression on first-line atezolizumab and bevacizumab (Atez/Bev) for advanced HCC remains controversial. The aim of this study was to analyze the real-world clinical results of second-line treatments in progression after Atez/Bev and to determine the factors affecting prognosis.
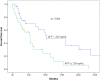
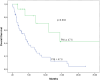
Materials and methods: Fifty-eight patients treated with second-line sorafenib, regorafenib, and cabozantinib for progression after first-line Atez/Bev for advanced/metastatic HCC from 20 centers in Türkiye between October 2020 and June 2024 were retrospectively analyzed. Responses were evaluated by Response criteria, specifically Response Evaluation Criteria in Solid Tumors (RECIST v1.1) criteria. Median overall survival (OS) and progression-free survival (PFS) were computed with the Kaplan-Meier method. The Cox regression model was utilized to analyze multivariate analyses.
Results: About 82.8% of the patients were male and the median age of the whole group was 62 (range, 18-78) years. All patients progressed after first-line Atez/Bev and were given second-line treatment. The most commonly used second-line treatment option was sorafenib (70.7%), followed by regorafenib (12.1%) and cabozantinib (10.3%). Both median PFS (4.1 months) and median OS (7.8 months) were longer in patients treated with sorafenib compared to other treatments. In univariate analyses, Child-Pugh score B, high alpha-fetoprotein (AFP) levels (>200 ng/mL), extrahepatic spread, and Prognostic Nutritional Index (PNI) < 47.6 substantially raised the risk of overall mortality. Multivariate analysis showed that extrahepatic spread (HR (Hazard ratio): 0.41, P = .012), PNI level (HR: 0.24, P = .005), and AFP level (HR:1.97, P = .049) were independent predictors of OS.
Conclusion: Although second-line therapies after Atez/Bev show different degrees of efficacy, survival rates are consistent with the literature. Extrahepatic spread, AFP level, and PNI level are the main prognostic factors. In light of this information, personalized treatment strategies may improve outcomes for this challenging patient group.
Keywords: Atezolizumab; bevacizumab; cabozantinib; hepatocellular carcinoma; overall survival; progression-free survival; regorafenib; second-line treatment; sorafenib.
Conflict of interest statement
Figures
Similar articles
-
The prognostic factors in patients with advanced hepatocellular carcinoma: impact of treatment sequencing.J Chemother. 2024 Nov;36(7):613-621. doi: 10.1080/1120009X.2024.2305066. Epub 2024 Jan 23. J Chemother. 2024. PMID: 38263804
-
Lenvatinib versus sorafenib as second-line therapy following progression on atezolizumab-bevacizumab in patients with unresectable hepatocellular carcinoma: a multicenter retrospective study from Korea and Japan.J Cancer Res Clin Oncol. 2025 Jan 28;151(2):52. doi: 10.1007/s00432-025-06085-1. J Cancer Res Clin Oncol. 2025. PMID: 39875560 Free PMC article.
-
Lenvatinib versus Sorafenib Second-Line Therapy in Patients with Hepatocellular Carcinoma Progressed to Atezolizumab plus Bevacizumab: A Retrospective Real-World Study.Oncology. 2025;103(6):456-468. doi: 10.1159/000541018. Epub 2024 Oct 11. Oncology. 2025. PMID: 39396495
-
Comparative efficacy and safety for second-line treatment with ramucirumab, regorafenib, and cabozantinib in patients with advanced hepatocellular carcinoma progressed on sorafenib treatment: A network meta-analysis.Medicine (Baltimore). 2021 Sep 24;100(38):e27013. doi: 10.1097/MD.0000000000027013. Medicine (Baltimore). 2021. PMID: 34559096 Free PMC article.
-
Is atezolizumab plus bevacizumab as first-line therapy for unresectable hepatocellular carcinoma superior to lenvatinib? a systematic review and meta‑analysis.Eur J Clin Pharmacol. 2024 Oct;80(10):1425-1434. doi: 10.1007/s00228-024-03718-1. Epub 2024 Jun 22. Eur J Clin Pharmacol. 2024. PMID: 38907884
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
