Single-molecule observations of human small heat shock proteins in complex with aggregation-prone client proteins
- PMID: 40241479
- PMCID: PMC12203938
- DOI: 10.1042/BCJ20240473
Single-molecule observations of human small heat shock proteins in complex with aggregation-prone client proteins
Abstract
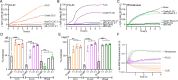
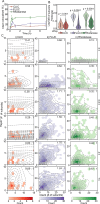
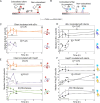
Small heat shock proteins (sHsps) are molecular chaperones that act to prevent the aberrant aggregation of misfolded proteins. Whilst it is suggested that sHsps prevent aggregation by binding to misfolded client proteins, the dynamic and heterogeneous nature of sHsps has hindered attempts to establish the mechanistic details of how sHsp-client protein complexes form. Single-molecule approaches have emerged as a powerful tool to investigate dynamic and heterogeneous interactions such as those that can occur between sHsps and their client proteins. Here, we use total internal reflection fluorescence microscopy to observe and characterise the complexes formed between model aggregation-prone client proteins (firefly luciferase, rhodanese and chloride intracellular channel 1 protein), and the human sHsps αB-crystallin (αB-c; HSPB5) and Hsp27 (HSPB1). We show that small (monomeric or dimeric) forms of both αB-c and Hsp27 bind to misfolded or oligomeric forms of the client proteins at early stages of aggregation, resulting in the formation of soluble sHsp-client complexes. Stoichiometric analysis of these complexes revealed that additional αB-c subunits accumulate onto pre-existing sHsp-client complexes to form larger species - this does not occur to the same extent for Hsp27. Instead, Hsp27-client interactions tend to be more transient than those of αB-c. Elucidating these mechanisms of sHsp function is crucial to our understanding of how these molecular chaperones act to inhibit protein aggregation and maintain cellular proteostasis.
Keywords: Hsp27; molecular chaperones; photobleaching; proteostasis; single-molecule fluorescence; αB-crystallin.
© 2025 The Author(s).
Conflict of interest statement
The authors declare that there are no conflicts associated with the manuscript.
Figures

Similar articles
-
O-GlcNAc modification of HSP27 alters its protein interactions and promotes refolding of proteins through the BAG3/HSP70 co-chaperone.Protein Sci. 2024 Oct;33(10):e5173. doi: 10.1002/pro.5173. Protein Sci. 2024. PMID: 39291732 Free PMC article.
-
The small heat shock proteins αB-crystallin (HSPB5) and Hsp27 (HSPB1) inhibit the intracellular aggregation of α-synuclein.Cell Stress Chaperones. 2017 Jul;22(4):589-600. doi: 10.1007/s12192-017-0785-x. Epub 2017 Mar 23. Cell Stress Chaperones. 2017. PMID: 28337642 Free PMC article.
-
Chaperone activity of human small heat shock protein-GST fusion proteins.Cell Stress Chaperones. 2017 Jul;22(4):503-515. doi: 10.1007/s12192-017-0764-2. Epub 2017 Jan 27. Cell Stress Chaperones. 2017. PMID: 28130664 Free PMC article.
-
EORTC guidelines for the use of erythropoietic proteins in anaemic patients with cancer: 2006 update.Eur J Cancer. 2007 Jan;43(2):258-70. doi: 10.1016/j.ejca.2006.10.014. Epub 2006 Dec 19. Eur J Cancer. 2007. PMID: 17182241
-
One size does not fit all: the oligomeric states of αB crystallin.FEBS Lett. 2013 Apr 17;587(8):1073-80. doi: 10.1016/j.febslet.2013.01.021. Epub 2013 Jan 20. FEBS Lett. 2013. PMID: 23340341 Free PMC article. Review.
Cited by
-
Mechanism of small heat shock protein client sequestration and induced polydispersity.Nat Commun. 2025 Apr 16;16(1):3635. doi: 10.1038/s41467-025-58964-3. Nat Commun. 2025. PMID: 40240363 Free PMC article.
-
Mechanism of small heat shock protein client sequestration and induced polydispersity.bioRxiv [Preprint]. 2024 Dec 6:2024.12.03.626640. doi: 10.1101/2024.12.03.626640. bioRxiv. 2024. Update in: Nat Commun. 2025 Apr 16;16(1):3635. doi: 10.1038/s41467-025-58964-3. PMID: 39677757 Free PMC article. Updated. Preprint.
References
-
- Ecroyd H., Bartelt-Kirbach B., Ben-Zvi A., Bonavita R., Bushman Y., Casarotto E., et al. The beauty and complexity of the small heat shock proteins: a report on the proceedings of the fourth workshop on small heat shock proteins. Cell Stress Chaperones. 2023;28:621–629. doi: 10.1007/s12192-023-01360-x. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

