Saturated phosphatidic acids induce mTORC1-driven integrated stress response contributing to glucolipotoxicity in hepatocytes
- PMID: 40241617
- PMCID: PMC12173470
- DOI: 10.1152/ajpgi.00027.2025
Saturated phosphatidic acids induce mTORC1-driven integrated stress response contributing to glucolipotoxicity in hepatocytes
Abstract
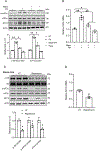
Hepatic glucolipotoxicity, characterized by the synergistic detrimental effects of elevated glucose levels combined with excessive lipid accumulation in hepatocytes, plays a central role in the pathogenesis of various metabolic liver diseases. Despite recent advancements, the precise mechanisms underlying this process remain unclear. Using cultured AML12 and HepG2 cells exposed to excess palmitate, with and without high glucose, as an in vitro model, we aimed to elucidate the cellular and molecular mechanisms underlying hepatic glucolipotoxicity. Our data showed that palmitate exposure induced the integrated stress response (ISR) in hepatocytes, evidenced by increased eukaryotic translation initiation factor 2 alpha (eIF2α) phosphorylation (serine 51) and upregulated activating transcription factor 4 (ATF4) expression. Moreover, we identified mammalian target of rapamycin complex 1 (mTORC1) as a novel upstream kinase responsible for palmitate-triggered ISR induction. Furthermore, we showed that either mTORC1 inhibitors, ISRIB (an ISR inhibitor), or ATF4 knockdown abolished palmitate-induced cell death, indicating that the mTORC1-eIF2α-ATF4 pathway activation plays a mechanistic role in mediating palmitate-induced hepatocyte cell death. Our continuous investigations revealed that glycerol-3-phosphate acyltransferase (GPAT4)-mediated metabolic flux of palmitate into the glycerolipid synthesis pathway is required for palmitate-induced mTORC1 activation and subsequent ISR induction. Specifically, we uncovered that saturated phosphatidic acid production contributes to palmitate-triggered mTORC1 activation. Our study provides the first evidence that high glucose enhances palmitate-induced activation of the mTORC1-eIF2α-ATF4 pathway, thereby exacerbating palmitate-induced hepatotoxicity. This effect is mediated by the increased availability of glycerol-3-phosphate, a substrate essential for phosphatidic acid synthesis. In conclusion, our study highlights that the activation of the mTORC1-eIF2α-ATF4 pathway, driven by saturated phosphatidic acid overproduction, plays a mechanistic role in hepatic glucolipotoxicity.NEW & NOTEWORTHY Integrated stress response (ISR) activation contributes to palmitate-induced lipotoxicity in hepatocytes. mTORC1 acts as an upstream kinase essential for palmitate-mediated ISR activation and hepatocyte death. The formation of saturated phosphatidic acid mechanistically regulates hepatic mTORC1 activation induced by palmitate. Glucose-enhanced generation of saturated phosphatidic acid amplifies palmitate-induced hepatotoxicity, contributing to glucolipotoxicity.
Keywords: ISR; glucolipotoxicity; mTORC1; palmitate; phosphatidic acid.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures





Similar articles
-
Increasing cellular NAD+ protects hepatocytes against palmitate-induced lipotoxicity by preventing PARP-1 inhibition and the mTORC1-p300 pathway activation.Am J Physiol Cell Physiol. 2025 Mar 1;328(3):C776-C790. doi: 10.1152/ajpcell.00946.2024. Epub 2025 Jan 27. Am J Physiol Cell Physiol. 2025. PMID: 39871470 Free PMC article.
-
Nicotinamide N-methyltransferase upregulation via the mTORC1-ATF4 pathway activation contributes to palmitate-induced lipotoxicity in hepatocytes.Am J Physiol Cell Physiol. 2021 Sep 1;321(3):C585-C595. doi: 10.1152/ajpcell.00195.2021. Epub 2021 Aug 11. Am J Physiol Cell Physiol. 2021. PMID: 34378991 Free PMC article.
-
Integrated Stress Response Potentiates Ponatinib-Induced Cardiotoxicity.Circ Res. 2024 Mar;134(5):482-501. doi: 10.1161/CIRCRESAHA.123.323683. Epub 2024 Feb 7. Circ Res. 2024. PMID: 38323474 Free PMC article.
-
ISR Modulators in Neurological Diseases.Curr Neuropharmacol. 2025;23(10):1184-1214. doi: 10.2174/011570159X361653250213114821. Curr Neuropharmacol. 2025. PMID: 39995125 Review.
-
Survival strategies of cancer cells: the role of macropinocytosis in nutrient acquisition, metabolic reprogramming, and therapeutic targeting.Autophagy. 2025 Apr;21(4):693-718. doi: 10.1080/15548627.2025.2452149. Epub 2025 Jan 26. Autophagy. 2025. PMID: 39817564 Free PMC article. Review.
Cited by
-
Saturated lipid stress attenuates mitochondrial genome synthesis in human cells.bioRxiv [Preprint]. 2025 Jul 29:2025.07.28.667051. doi: 10.1101/2025.07.28.667051. bioRxiv. 2025. PMID: 40766347 Free PMC article. Preprint.
References
-
- Prentki M, Segall L, Roche E, Thumelin S, Brun T, McGarry JD, Corkey BE, and Assimacopoulos-Jeannet F. [Gluco-lipotoxicity and gene expression in the pancreatic beta cell]. Journ Annu Diabetol Hotel Dieu 17–27, 1998. - PubMed
-
- Unger RH, Clark GO, Scherer PE, and Orci L. Lipid homeostasis, lipotoxicity and the metabolic syndrome. Biochim Biophys Acta 1801: 209–214, 2010. - PubMed
-
- Kuchay MS, Choudhary NS, and Mishra SK. Pathophysiological mechanisms underlying MAFLD. Diabetes Metab Syndr 14: 1875–1887, 2020. - PubMed
-
- Lee JY, Cho HK, and Kwon YH. Palmitate induces insulin resistance without significant intracellular triglyceride accumulation in HepG2 cells. Metabolism 59: 927–934, 2010. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

