An Interplay Between Pericytes, Mesenchymal Stem Cells, and Immune Cells in the Process of Tissue Regeneration
- PMID: 40241723
- PMCID: PMC12003036
- DOI: 10.1155/ancp/4845416
An Interplay Between Pericytes, Mesenchymal Stem Cells, and Immune Cells in the Process of Tissue Regeneration
Abstract
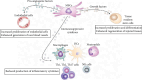
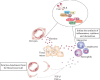
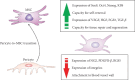
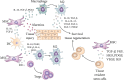
Immediately after injury, damaged cells elicit tissue regeneration, a healing process that enables optimal renewal and regrowth of injured tissues. Results obtained in a large number of experimental studies suggested that the cross talk between pericytes, mesenchymal stem cells (MSC), tissue-resident stem cells, and immune cells has a crucially important role in the regeneration of injured tissues. Pericytes, MSCs, and immune cells secrete bioactive factors that influence each other's behavior and function. Immune cells produce inflammatory cytokines and chemokines that influence pericytes' migration, proliferation, and transition to MSC. MSC releases immunoregulatory factors that induce the generation of immunosuppressive phenotype in inflammatory immune cells, alleviating detrimental immune responses in injured tissues. MSC also produces various growth factors that influence the differentiation of tissue-resident stem cells into specific cell lineages, enabling the successful regeneration of injured tissues. A better understanding of molecular mechanisms that regulate crosstalk between pericytes, MSC, and immune cells in injured tissues would enable the design of new therapeutic approaches in regenerative medicine. Accordingly, in this review paper, we summarized current knowledge related to the signaling pathways that are involved in the pericytes' activation, pericytes-to-MSC transition, differentiation of tissue-resident stem cells, and MSC-dependent modulation of immune cell-driven inflammation, which are crucially responsible for regeneration of injured tissues.
Keywords: exosomes; immune cells; mesenchymal stem cells; pericytes; tissue regeneration; tissue-resident stem cells.
Copyright © 2025 Vladislav Volarevic et al. Analytical Cellular Pathology published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
The pericyte secretome: Potential impact on regeneration.Biochimie. 2018 Dec;155:16-25. doi: 10.1016/j.biochi.2018.04.015. Epub 2018 Apr 23. Biochimie. 2018. PMID: 29698670 Review.
-
Perivascular-Derived Mesenchymal Stem Cells.J Dent Res. 2019 Sep;98(10):1066-1072. doi: 10.1177/0022034519862258. Epub 2019 Jul 5. J Dent Res. 2019. PMID: 31276626 Review.
-
Mesenchymal Stem Cell-Derived Exosomes and Other Extracellular Vesicles as New Remedies in the Therapy of Inflammatory Diseases.Cells. 2019 Dec 11;8(12):1605. doi: 10.3390/cells8121605. Cells. 2019. PMID: 31835680 Free PMC article. Review.
-
NLRP3 Inflammasome as a Potentially New Therapeutic Target of Mesenchymal Stem Cells and Their Exosomes in the Treatment of Inflammatory Eye Diseases.Cells. 2023 Sep 21;12(18):2327. doi: 10.3390/cells12182327. Cells. 2023. PMID: 37759549 Free PMC article. Review.
-
Human Glioblastoma-Derived Mesenchymal Stem Cell to Pericytes Transition and Angiogenic Capacity in Glioblastoma Microenvironment.Cell Physiol Biochem. 2018;46(1):279-290. doi: 10.1159/000488429. Epub 2018 Mar 22. Cell Physiol Biochem. 2018. PMID: 29590646
References
-
- Ruan Q., Tan S., Guo L., Ma D., Wen J. Prevascularization Techniques for Dental Pulp Regeneration: Potential Cell Sources, Intercellular Communication and Construction Strategies. Frontiers in Bioengineering and Biotechnology . 2023;11:p. 1186030. doi: 10.3389/fbioe.2023.1186030. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

