Maternal transfer of anti-GAPDH IgG prevents neonatal infections caused by Staphylococcus aureus and group B Streptococcus
- PMID: 40241760
- PMCID: PMC12002998
- DOI: 10.1016/j.isci.2025.112248
Maternal transfer of anti-GAPDH IgG prevents neonatal infections caused by Staphylococcus aureus and group B Streptococcus
Abstract
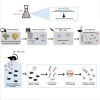
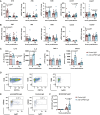
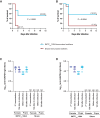
Group B Streptococcus (GBS) and Staphylococcus aureus cause 200.000 neonatal deaths every year and no vaccine has been developed yet. Here, we described that extracellular glyceraldehyde-3-phosphate dehydrogenase (GAPDH) from S. aureus is an immunomodulatory protein. Antibody mediated neutralization of S. aureus extracellular GAPDH promotes a protective inflammatory response by inhibiting an early and abnormal production of IL-10 in infected neonatal mice. As an immunomodulatory role for extracellular GAPDH was already described for GBS, we selected peptides exposed on bacterial GAPDH from both bacteria but completely absent from human GAPDH. These peptides were chemically synthesized and conjugated to a carrier protein. Maternal vaccination with these conjugated peptides induced an increased survival of mouse pups from infection with GBS or S. aureus, when compared to controls. The addition of anti-bacterial GAPDH IgG into infected human cord-blood cells caused a significant reduction in bacterial replication, suggesting a putative efficacy for humans.
Keywords: Immunology; Microbiology.
© 2025 The Author(s).
Conflict of interest statement
P.M. is the author of a patent related to this work (PCT/EP2015/063243). All the rights on IMTPvac1804 and on different forms to block GAPDH-mediated virulence mechanism are Immunethep property. F.L., M.V., A.F., L.C., C.N., J.B.N., P.C., C.F., C.T., and P.M. are Immunethep employees. All the other authors declare no financial or non-financial competing interests.
Figures




Similar articles
-
Inhibition of IL-10 production by maternal antibodies against Group B Streptococcus GAPDH confers immunity to offspring by favoring neutrophil recruitment.PLoS Pathog. 2011 Nov;7(11):e1002363. doi: 10.1371/journal.ppat.1002363. Epub 2011 Nov 17. PLoS Pathog. 2011. PMID: 22114550 Free PMC article.
-
Maternal vaccination against group B Streptococcus glyceraldehyde-3-phosphate dehydrogenase leads to gut dysbiosis in the offspring.Brain Behav Immun. 2022 Jul;103:186-201. doi: 10.1016/j.bbi.2022.04.004. Epub 2022 Apr 12. Brain Behav Immun. 2022. PMID: 35427758
-
A Safe and Stable Neonatal Vaccine Targeting GAPDH Confers Protection against Group B Streptococcus Infections in Adult Susceptible Mice.PLoS One. 2015 Dec 16;10(12):e0144196. doi: 10.1371/journal.pone.0144196. eCollection 2015. PLoS One. 2015. PMID: 26673420 Free PMC article.
-
Glyceradehyde-3-phosphate dehydrogenase as a suitable vaccine candidate for protection against bacterial and parasitic diseases.Vaccine. 2016 Feb 17;34(8):1012-7. doi: 10.1016/j.vaccine.2015.11.072. Epub 2015 Dec 10. Vaccine. 2016. PMID: 26686572 Review.
-
Aetiology of neonatal sepsis in Nigeria, and relevance of Group b streptococcus: A systematic review.PLoS One. 2018 Jul 17;13(7):e0200350. doi: 10.1371/journal.pone.0200350. eCollection 2018. PLoS One. 2018. PMID: 30016358 Free PMC article.
References
-
- Mahtab S., Madhi S.A., Baillie V.L., Els T., Thwala B.N., Onyango D., Tippet-Barr B.A., Akelo V., Igunza K.A., Omore R., et al. Causes of death identified in neonates enrolled through Child Health and Mortality Prevention Surveillance (CHAMPS), December 2016 -December 2021. PLOS Glob. Public Health. 2023;3 doi: 10.1371/journal.pgph.0001612. - DOI - PMC - PubMed
-
- Goncalves B.P., Procter S.R., Paul P., Chandna J., Lewin A., Seedat F., Koukounari A., Dangor Z., Leahy S., Santhanam S., et al. Group B streptococcus infection during pregnancy and infancy: estimates of regional and global burden. Lancet Global Health. 2022;10:e807–e819. doi: 10.1016/S2214-109X(22)00093-6. - DOI - PMC - PubMed
-
- Droz N., Hsia Y., Ellis S., Dramowski A., Sharland M., Basmaci R. Bacterial pathogens and resistance causing community acquired paediatric bloodstream infections in low- and middle-income countries: a systematic review and meta-analysis. Antimicrob. Resist. Infect. Control. 2019;8:207. doi: 10.1186/s13756-019-0673-5. - DOI - PMC - PubMed
-
- Okomo U., Akpalu E.N.K., Le Doare K., Roca A., Cousens S., Jarde A., Sharland M., Kampmann B., Lawn J.E. Aetiology of invasive bacterial infection and antimicrobial resistance in neonates in sub-Saharan Africa: a systematic review and meta-analysis in line with the STROBE-NI reporting guidelines. Lancet Infect. Dis. 2019;19:1219–1234. doi: 10.1016/S1473-3099(19)30414-1. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

