CXCR4+ PD-L1+ neutrophils are increased in non-survived septic mice
- PMID: 40241761
- PMCID: PMC12003019
- DOI: 10.1016/j.isci.2025.112083
CXCR4+ PD-L1+ neutrophils are increased in non-survived septic mice
Abstract
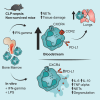
The dysregulated host response to infections can lead to sepsis, a complex disease characterized by a spectrum of clinical phenotypes. Using scRNA-seq, we analyzed the immune cell of survived and non-survived CLP-septic mice to gain insights into the immunological mechanisms by which neutrophils contribute to the hyperinflammatory phenotype. Our findings reveal that non-survived mice exhibit increased frequencies of immature CXCR4+ PD-L1+ neutrophils in the bloodstream, accompanied by an accumulation of trafficking-specific CXCR4+ PD-L1+ neutrophils into the lungs. The IFN-gamma and LPS promote the PD-L1 expression on neutrophils and an activation profile associated with inflammation and organ damage. Notably, abrogating the IFN-gamma reduced susceptibility to CLP-sepsis and diminished CXCR4+ PD-L1+ neutrophils frequency. This study provides insights into the immune cell activation profiles associated with the worsening of the CLP-sepsis, and the CXCR4+ PD-L1+ neutrophils population highlighted here represents a promising target for therapeutic modulation in clinical sepsis hyperinflammatory phenotype.
Keywords: Biological sciences; Immune response; Immunology; Natural sciences.
© 2025 The Author(s).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
-
- Singer M., Deutschman C.S., Seymour C.W., Shankar-Hari M., Annane D., Bauer M., Bellomo R., Bernard G.R., Chiche J.-D., Coopersmith C.M., et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. - DOI - PMC - PubMed
-
- Davenport E.E., Burnham K.L., Radhakrishnan J., Humburg P., Hutton P., Mills T.C., Rautanen A., Gordon A.C., Garrard C., Hill A.V.S., et al. Genomic landscape of the individual host response and outcomes in sepsis: a prospective cohort study. Lancet Respir. Med. 2016;4:259–271. doi: 10.1016/S2213-2600(16)00046-1. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

