Heat shock affects the Ca2+/calmodulin-dependent protein kinase II dynamic during bovine sperm capacitation and acrosome reaction
- PMID: 40241796
- PMCID: PMC12001034
- DOI: 10.3389/fcell.2025.1552282
Heat shock affects the Ca2+/calmodulin-dependent protein kinase II dynamic during bovine sperm capacitation and acrosome reaction
Abstract
Background: Heat shock during sperm capacitation affects the spermatozoa quality, resulting in increased early acrosome reaction and consequently decreasing their fertilizing capacity. Although the mechanisms involved in the regulation of sperm capacitation and acrosome reaction are not fully understood, it has been reported that Ca2+/calmodulin-dependent protein kinase II (CaMKII) is an important regulator of these processes. Thus, the present aimed to evaluate the effect of heat shock in the CaMKII signaling during the bovine sperm capacitation and acrosome.
Methods: Bovine spermatozoa were in vitro capacitated for 4 hours. The acrosome reaction was induced by exposure to heparin and calcium ionophore A23187 for 1 hour. Heat shock was applied by incubating spermatozoa at 41 °C with 7% CO2, while the control group was maintained at 38.5 °C with 5% CO2. At the end of each treatment, the localization of total CaMKII and phosphorylated CaMKII (pCaMKII), as well as acrosomal membrane integrity, were evaluated by immunofluorescence.
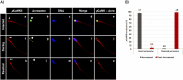
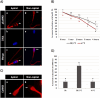
Results: It was observed that CaMKII and not phosphorylated CaMKII (pCaMKII) localization at the acrosome region was affected by sperm capacitation. In contrast, the localization of both, CaMKII and its phosphorylated form was affected by the acrosome reaction (p < 0.05). The acrosome membrane integrity, as well as the pCamKII localization in bovine spermatozoa, was affected by incubation time. This effect of incubation time was stronger in heated shock sperm, although it was observed only after 2 h of incubation. Heat shock also affected the acrosomal localization of pCaMKII in the acrosomal region of spermatozoa with intact acrosome.
Discussion: Taken together, the data present here show that CaMKII and pCaMKII localization is dynamic during bovine sperm capacitation and acrosome reaction and that this pattern of localization is affected by heat shock, suggesting that failure in CaMKII signaling is probably involved in the early acrosome reaction observed in heated-shock spermatozoa.
Keywords: acrosomal membrane; environment; heat stress; phosphorylation; spermatozoa.
Copyright © 2025 Santos, Contrim, da Silva, Assumpção, Paula-Lopes and Feitosa.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Changes in calmodulin immunocytochemical localization associated with capacitation and acrosomal exocytosis of ram spermatozoa.Theriogenology. 2009 Mar 15;71(5):789-800. doi: 10.1016/j.theriogenology.2008.10.003. Epub 2008 Dec 9. Theriogenology. 2009. PMID: 19081128
-
Are capacitation or calcium ion influx required for the human sperm acrosome reaction?Fertil Steril. 1994 Dec;62(6):1255-61. doi: 10.1016/s0015-0282(16)57195-7. Fertil Steril. 1994. PMID: 7957994
-
Changes in content and localization of proteins phosphorylated at tyrosine, serine and threonine residues during ram sperm capacitation and acrosome reaction.Reproduction. 2009 Apr;137(4):655-67. doi: 10.1530/REP-08-0280. Epub 2009 Jan 16. Reproduction. 2009. PMID: 19151126
-
The Presence of Seminal Plasma during Liquid Storage of Pig Spermatozoa at 17 °C Modulates Their Ability to Elicit In Vitro Capacitation and Trigger Acrosomal Exocytosis.Int J Mol Sci. 2020 Jun 25;21(12):4520. doi: 10.3390/ijms21124520. Int J Mol Sci. 2020. PMID: 32630462 Free PMC article.
-
Intracellular events and signaling pathways involved in sperm acquisition of fertilizing capacity and acrosome reaction.Front Biosci. 2000 Nov 1;5:E110-23. doi: 10.2741/baldi. Front Biosci. 2000. PMID: 11056077 Review.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

