Incidence of upper respiratory tract infections with biological therapies in moderate to severe atopic dermatitis: a systematic review and meta-analysis
- PMID: 40241901
- PMCID: PMC12000152
- DOI: 10.3389/fmed.2025.1550640
Incidence of upper respiratory tract infections with biological therapies in moderate to severe atopic dermatitis: a systematic review and meta-analysis
Abstract
Introduction: Atopic dermatitis (AD) is a chronic inflammatory skin condition affecting 5%-20% of children and 2%-10% of adults worldwide. Treatment for moderate-to-severe AD includes biologics like dupilumab, tralokinumab, lebrikizumab, and JAK inhibitors (abrocitinib, upadacitinib). However, upper respiratory tract infections (URTIs) are commonly reported adverse events for these therapies. This meta-analysis aims to estimate the pooled incidence of URTIs associated with these treatments compared to topicals.
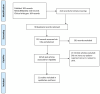
Methods: A systematic search was conducted across PubMed, MEDLINE, DOAJ, and ClinicalTrials.gov for randomized controlled trials (RCTs) involving AD patients treated with dupilumab, tralokinumab, lebrikizumab, abrocitinib, or upadacitinib, excluding studies of patients treated with topicals, Studies on other dermatitis types and biologics. Data on URTI events, sample sizes, and incidence were extracted. Study quality was assessed using the Cochrane Risk of Bias Tool (RoB 2). A random-effects meta-analysis was conducted using the Netmeta package in R, calculating odds ratios (ORs) with 95% confidence intervals (CIs).
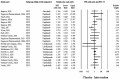
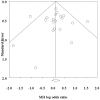
Results: From 413 retrieved records, 21 studies met the inclusion criteria. URTI incidence of the treatment group in the included studies ranged from 0.35% to 41.5%, while control groups showed rates between 0% and 40%. Across all studies, URTI incidence was 9.70% in intervention groups and 8.03% in placebo groups (MH OR = 1.18, 95% CI: 0.98-1.42). Heterogeneity was low (I 2 = 20.14%), with no evidence of publication bias (p = 0.83). There were no significant subgroup differences between patients taking different biological therapies (Q = 3.90, p = 0.42).
Conclusion: While URTIs are common adverse events for AD therapies, their incidence in intervention groups is similar to control, suggesting no significant increase in risk. These findings provide critical insights for clinicians in balancing efficacy and safety when selecting therapies for AD patients. Further research should explore patient-specific risk factors for URTIs.
Systematic review registration: Prospero registration code: [392093]. PROSPERO, Centre for Reviews and Dissemination: CRD42023392093.
Keywords: IL13 inhibitors; IL4/13 inhibitor; abrocitinib; atopic dermatitis; biologics; dupilumab; lebrikizumab; tralokinumab.
Copyright © 2025 Alraddadi, Kalantan, Aljefri, Maaddawi, Alsamadani, Kadasa, Softah, Tabbakh, Alturkistani and Jfri.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

