Assessment of readability of informed consent forms of gynecologic cancer clinical trials: A (Potential) barrier to enrollment?
- PMID: 40241924
- PMCID: PMC12002828
- DOI: 10.1016/j.gore.2025.101730
Assessment of readability of informed consent forms of gynecologic cancer clinical trials: A (Potential) barrier to enrollment?
Abstract
Objective: Limited English language proficiency (LEP) is associated with decreased clinical trial enrollment. Notably, the AMA and the NIH recommend that patient materials have a readability level commensurate to a sixth- through eighth-grade reading level. This study evaluated the readability of informed consent forms for gynecologic oncology clinical trials.
Methods: This was an IRB-exempt, retrospective, quantitative analysis of for gynecologic oncology clinical trials opened at Ohio State University, a National Cancer Institute (NCI)-designated institution, from 1/1/2017 through 12/31/2022. We analyzed patient informed consent documents from gynecologic oncology clinical trials. The researchers assessed the readability of these consent forms using standardized readability tests to determine their complexity and readability levels. Readability was assessed using Readability Studio Professional Edition software for five metrics.
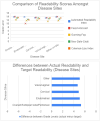
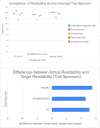
Results: A total of 103 informed consent forms were reviewed, capturing trials for ovarian (n = 41, 39.8 %), endometrial (n = 21, 20.4 %), cervical (n = 14, 13.6 %), vulvar/vaginal cancers (n = 3, 2.9 %), as well as multi-disease site/basket trials (n = 24, 23.3 %). Most informed consent forms were from industry-sponsored studies (n = 45, 43.7 %) and NCI, NRG Oncology, and GOG Foundation (GOG) sponsored studies (n = 42, 40.8 %). The mean reading grade-level for all analyses was 13th grade, specifically 13 for ovarian cancer, 12.02 for endometrial, 12.9 for cervical, 12.8 for vulva/vaginal, and 13.0 for others (p = 0.26). There was no difference (p = 0.21) between NCI/NRG/GOG studies (13.3) and industry-sponsored trials (13.6).
Conclusions: In this study, we found that current informed consent forms do not meet current recommended readability standards for medical literature regardless of disease site or sponsor. This is an opportunity to reduce disparities and improve patient understanding and involvement in clinical trials.
Keywords: Cervical cancer; Clinical trial enrollment; Endometrial cancer; Gynecologic cancer; Literacy; Ovarian cancer; Readability.
© 2025 The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- Clinical Trials. (n.d.). NCCN. [Accessed 25 Feb 2025}. Available from: https://www.nccn.org/patientresources/patient-resources/support-for-pati....
-
- Medina S.P., et al. Experiences of a multiethnic cohort of patients enrolled in a financial reimbursement program for cancer clinical trials. JCO Oncol Pract [online] 2023;19(5) doi: 10.1200/OP.22.00429. https://ascopubs.org/doi/10.1200/OP.22.00429?url_ver=Z39.88-2003&rfr_id=... e801-e810 Epub 2023 Feb 17. [Accessed 25 Feb 2025}. Available from: - DOI - DOI - PubMed
-
- Reopell L., et al. Community engagement and clinical trial diversity: Navigating barriers and co-designing solutions-A report from the “Health Equity through Diversity” seminar series. PLoS One[online] 2023;18(2) https://pubmed.ncbi.nlm.nih.gov/36795792/ [Accessed 25 Feb 2025}. Available from: - PMC - PubMed
-
- Jorge S., et al. Participation of patients with limited english proficiency in gynecologic oncology clinical trials. J. Natl. Compr. Canc. Netw. [online] 2023;21(1) https://jnccn.org/view/journals/jnccn/21/1/article-p27.xml 27 32.e2 [Accessed 25 Feb 2025}. Available from: - PubMed
-
- Gupta U.C. Informed consent in clinical research: revisiting few concepts and areas. Perspect Clin Res [online] 2013;4(1):26–32. https://pmc.ncbi.nlm.nih.gov/articles/pmid/23533976/ [Accessed 25 Feb 2025}. Available from: - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
