Omalizumab is ineffective in regulating proasthmatic serum cytokine and chemokine levels in nonresponders with high BMI
- PMID: 40242148
- PMCID: PMC12002203
- DOI: 10.1016/j.jacig.2025.100462
Omalizumab is ineffective in regulating proasthmatic serum cytokine and chemokine levels in nonresponders with high BMI
Abstract
Background: Omalizumab provides clinical benefits to a fraction of patients with asthma. It remains unclear why some patients do not respond to omalizumab therapy.
Objective: We sought to investigate whether omalizumab could alter serum cytokine and chemokine levels that could be associated with asthma pathogenesis. We also investigated why omalizumab is ineffective in controlling proasthmatic serum cytokine and chemokine levels in nonresponders.
Methods: Serum cytokine and chemokine levels in patients with moderate to severe asthma (N = 45; 34 responders and 11 nonresponders) were assessed before and after 26 weeks of omalizumab therapy. Correlations between cytokine and chemokine levels and asthma symptoms as well as characteristics of responders and nonresponders were assessed. Nonasthmatic subjects (N = 22) served as controls for patients with asthma (N = 45).
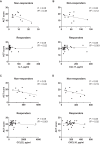
Results: Omalizumab was more effective in patients with increased serum eotaxin-1 and IL-13 levels than in others at baseline. Omalizumab decreased eotaxin-1 and IL-13, along with levels of most of the cytokines and chemokines tested, including IL-7, CCL17, and CXCL10, in responders, except for CCL5 and CCL22, which can contribute to neutrophilic and type 2 airway inflammation, respectively. In contrast, omalizumab did not decrease such serum cytokine and chemokine levels in nonresponders. Of interest, serum CCL17, CCL22, CXCL10, and IL-7 levels in nonresponders were associated with their body mass index, which could explain why omalizumab was unable to reduce their concentrations in nonresponders.
Conclusions: Omalizumab can regulate most cytokine and chemokine levels in responders. However, in nonresponders, it is unable to modulate specific proasthmatic cytokines and chemokines due to their association with individual body mass index, which is not influenced by omalizumab.
Keywords: Asthma; IgE; anti-IgE; body mass index; chemokine; cytokine; omalizumab; serum.
© 2025 The Author(s).
Conflict of interest statement
This study was supported by the 10.13039/100008925American Asthma Foundation (grant no. AAF15-0038 to S.O.), Investigator-Initiated Study from Genentech (grant no. ML28019 to S.O.), and Mayo Clinic. Disclosure of potential conflict of interest: The authors declare that they have no relevant conflicts of interest.
Figures

Similar articles
-
Baseline serum CXCL10 and IL-12 levels may predict severe asthmatics' responsiveness to omalizumab.Respir Med. 2018 Jan;134:95-102. doi: 10.1016/j.rmed.2017.12.002. Epub 2017 Dec 5. Respir Med. 2018. PMID: 29413515 Clinical Trial.
-
Ratio of plasma IL-13/TNF- ∝ and CXCL10/CCL17 predicts mepolizumab and omalizumab response in asthma better than eosinophil count or immunoglobulin E level.Sci Rep. 2024 May 6;14(1):10404. doi: 10.1038/s41598-024-60864-3. Sci Rep. 2024. PMID: 38710930 Free PMC article.
-
Endotypes of severe allergic asthma patients who clinically benefit from anti-IgE therapy.Clin Exp Allergy. 2019 Jan;49(1):44-53. doi: 10.1111/cea.13248. Epub 2018 Sep 11. Clin Exp Allergy. 2019. PMID: 30107059 Clinical Trial.
-
Spotlight on omalizumab in allergic asthma.BioDrugs. 2004;18(6):415-8. doi: 10.2165/00063030-200418060-00007. BioDrugs. 2004. PMID: 15571425 Review.
-
Omalizumab: a review of its use in the management of allergic asthma.Treat Respir Med. 2004;3(3):183-99. doi: 10.2165/00151829-200403030-00006. Treat Respir Med. 2004. PMID: 15219177 Review.
References
-
- Holgate S., Casale T., Wenzel S., Bousquet J., Deniz Y., Reisner C. The anti-inflammatory effects of omalizumab confirm the central role of IgE in allergic inflammation. J Allergy Clin Immunol. 2005;115:459–465. - PubMed
-
- Holgate S.T., Djukanovic R., Casale T., Bousquet J. Anti-immunoglobulin E treatment with omalizumab in allergic diseases: an update on anti-inflammatory activity and clinical efficacy. Clin Exp Allergy. 2005;35:408–416. - PubMed
-
- Abraham I., Alhossan A., Lee C.S., Kutbi H., MacDonald K. ‘Real-life’ effectiveness studies of omalizumab in adult patients with severe allergic asthma: systematic review. Allergy. 2016;71:593–610. - PubMed
-
- Hanania N.A., Alpan O., Hamilos D.L., Condemi J.J., Reyes-Rivera I., Zhu J., et al. Omalizumab in severe allergic asthma inadequately controlled with standard therapy: a randomized trial. Ann Intern Med. 2011;154:573–582. - PubMed
-
- Chang T.W., Wu P.C., Hsu C.L., Hung A.F. Anti-IgE antibodies for the treatment of IgE-mediated allergic diseases. Adv Immunol. 2007;93:63–119. - PubMed
LinkOut - more resources
Full Text Sources

