Serum FGF21 as a predictor of response to atezolizumab and bevacizumab in HCC
- PMID: 40242310
- PMCID: PMC11999275
- DOI: 10.1016/j.jhepr.2025.101364
Serum FGF21 as a predictor of response to atezolizumab and bevacizumab in HCC
Abstract
Background & aims: Fibroblast growth factor 21 (FGF21) is a crucial regulator of cell metabolism. Tumour-secreted FGF21 has shown immune-checkpoint factor functions, and high FGF21 levels are associated with a poor prognosis for patients. However, its prognostic value and impact on treatment response in patients with hepatocellular carcinoma (HCC) treated with immune-checkpoint inhibitors (ICIs) remain unclear. Thus, this study investigated the potential of high FGF21 levels as a prognostic marker and whether traditional ICI-based therapy can improve the prognosis of patients with high FGF21 levels.
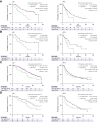
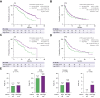
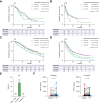
Methods: In this retrospective multicentre study, patients with unresectable HCC who received atezolizumab/bevacizumab in the NORTE study group (n = 117) were classified into high (≥915 pg/ml; n = 29) and non-high (n = 88) FGF21 groups. For validation, we investigated patients treated with atezolizumab/bevacizumab in an independent cohort (n = 285). Overall survival, progression-free survival, and treatment response were compared between patients with and without high baseline FGF21 levels.
Results: The median overall survival (p <0.001) and progression-free survival (p = 0.045) were significantly shorter in the high FGF21 group than in the non-high FGF21 group. Independent cohort analysis validated these results. In the overall cohort, the median progression-free survival (5.75 vs. 8.84 months; p = 0.027) and median overall survival (14.13 vs. 22.08 months; p <0.001) were significantly shorter in the high FGF21 group than in the non-high FGF21 group. The durable response (≥6 months) + complete response rate was significantly decreased in the high FGF21 group (p = 0.045). No patient with a high FGF21 level achieved a complete response, whereas this was achieved in 4.1% (13/319) of patients with non-high FGF21 levels. Multivariate Cox regression analysis identified high baseline serum FGF21 as an independent poor prognostic factor for overall survival (hazard ratio 2.20, p <0.001).
Conclusions: Serum FGF21 may be a robust, non-invasive prognostic and treatment response marker for unresectable HCC treated with atezolizumab/bevacizumab.
Impact and implications: FGF21 has been reported to act as a secreted immune-checkpoint factor, and elevated levels of FGF21 are associated with a poor prognosis in patients with HCC. It is not fully understood whether ICIs can overcome the impact of high FGF21 levels on the shortened prognosis of patients with HCC. In this multicentre retrospective study, patients with HCC and high baseline levels of serum FGF21 who received atezolizumab/bevacizumab treatment exhibited a significantly shorter overall survival and shorter progression-free survival. These findings suggest serum FGF21 as a robust prognostic marker and an indicator of treatment response in unresectable HCC treated with ICI-based therapy. These findings could be crucial for the implementation of personalised treatment strategies for unresectable HCC. However, identifying optimal therapeutic options for patients with unresectable HCC and high serum FGF21 levels remains an urgent and critical clinical issue.
Keywords: Atezolizumab; Bevacizumab; FGF21; Hepatocellular Carcinoma; Immune-checkpoint inhibitor; Prognosis.
© 2025 The Author(s).
Conflict of interest statement
NS received lecture fees from Chugai Pharmaceutical Co., and research grants from Gilead Sciences and AbbVie. GS received research grants from Gilead Sciences. AK received a research grant from Chugai Pharmaceutical Co.MK received honoraria for lectures from Eisai Co., Chugai Pharmaceutical Co., Astra Zeneca, Gilead Sciences, and AbbVie. KT received honoraria for lectures from Eisai Co., Chugai Pharmaceutical Co., Astra Zeneca, and Takeda Pharmaceuticals. YYasui received honoraria for lectures from Eisai Co., Chugai Pharmaceutical Co., AstraZeneca, and Eli Lilly. The remaining authors declare no conflicts of interest. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures

References
-
- Sung H., Ferlay J., Siegel R.L., et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. - PubMed
-
- Llovet J.M., Kelley R.K., Villanueva A., et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:6. - PubMed
-
- Yang X., Wang D., Lin J., et al. Atezolizumab plus bevacizumab for unresectable hepatocellular carcinoma. Lancet Oncol. 2020;21 - PubMed
-
- Abou-Alfa G.K., Lau G., Kudo M., et al. Tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. NEJM Evid. 2022;1 - PubMed
-
- Lewis J.E., Ebling F.J.P., Samms R.J., et al. Going back to the biology of FGF21: new insights. Trends Endocrinol Metab. 2019;30:491–504. - PubMed
LinkOut - more resources
Full Text Sources

