An engineered M2 macrophage-derived exosomes-loaded electrospun biomimetic periosteum promotes cell recruitment, immunoregulation, and angiogenesis in bone regeneration
- PMID: 40242509
- PMCID: PMC12002949
- DOI: 10.1016/j.bioactmat.2025.03.027
An engineered M2 macrophage-derived exosomes-loaded electrospun biomimetic periosteum promotes cell recruitment, immunoregulation, and angiogenesis in bone regeneration
Abstract
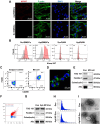
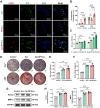
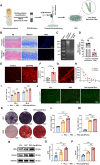
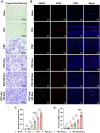
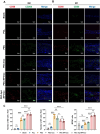
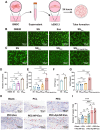
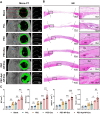
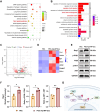
The periosteum, a fibrous tissue membrane covering bone surfaces, is critical to osteogenesis and angiogenesis in bone reconstruction. Artificial periostea have been widely developed for bone defect repair, but most of these are lacking of periosteal bioactivity. Herein, a biomimetic periosteum (termed PEC-Apt-NP-Exo) is prepared based on an electrospun membrane combined with engineered exosomes (Exos). The electrospun membrane is fabricated using poly(ε-caprolactone) (core)-periosteal decellularized extracellular matrix (shell) fibers via coaxial electrospinning, to mimic the fibrous structure, mechanical property, and tissue microenvironment of natural periosteum. The engineered Exos derived from M2 macrophages are functionalized by surface modification of bone marrow mesenchymal stem cell (BMSC)-specific aptamers to further enhance cell recruitment, immunoregulation, and angiogenesis in bone healing. The engineered Exos are covalently bonded to the electrospun membrane, to achieve rich loading and long-term effects of Exos. In vitro experiments demonstrate that the biomimetic periosteum promotes BMSC migration and osteogenic differentiation via Rap1/PI3K/AKT signaling pathway, and enhances vascular endothelial growth factor secretion from BMSCs to facilitate angiogenesis. In vivo studies reveal that the biomimetic periosteum promotes new bone formation in large bone defect repair by inducing M2 macrophage polarization, endogenous BMSC recruitment, osteogenic differentiation, and vascularization. This research provides valuable insights into the development of a multifunctional biomimetic periosteum for bone regeneration.
Keywords: Biomimetic periosteum; Bone regeneration; Engineered exosome; Immune regulation; Osteogenic induction.
© 2025 The Authors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Electrospun Biomimetic Periosteum Promotes Diabetic Bone Defect Regeneration through Regulating Macrophage Polarization and Sequential Drug Release.ACS Biomater Sci Eng. 2025 Mar 10;11(3):1690-1704. doi: 10.1021/acsbiomaterials.4c02095. Epub 2025 Feb 5. ACS Biomater Sci Eng. 2025. PMID: 39908041
-
Bioinspired Piezoelectric Periosteum to Augment Bone Regeneration via Synergistic Immunomodulation and Osteogenesis.ACS Appl Mater Interfaces. 2023 Mar 8;15(9):12273-12293. doi: 10.1021/acsami.2c19767. Epub 2023 Feb 22. ACS Appl Mater Interfaces. 2023. PMID: 36890691
-
Biomimetic periosteum combining BMP-2-loaded M2 macrophage-derived exosomes for enhanced bone defect repair.Front Bioeng Biotechnol. 2025 Aug 1;13:1639394. doi: 10.3389/fbioe.2025.1639394. eCollection 2025. Front Bioeng Biotechnol. 2025. PMID: 40821662 Free PMC article.
-
Extracellular vesicle-loaded hydrogels for tissue repair and regeneration.Mater Today Bio. 2022 Dec 21;18:100522. doi: 10.1016/j.mtbio.2022.100522. eCollection 2023 Feb. Mater Today Bio. 2022. PMID: 36593913 Free PMC article. Review.
-
Periosteum and development of the tissue-engineered periosteum for guided bone regeneration.J Orthop Translat. 2022 Feb 16;33:41-54. doi: 10.1016/j.jot.2022.01.002. eCollection 2022 Mar. J Orthop Translat. 2022. PMID: 35228996 Free PMC article. Review.
References
-
- Allen M.R., Hock J.M., Burr D.B. Periosteum: biology, regulation, and response to osteoporosis therapies. Bone. 2004;35(5):1003–1012. - PubMed
-
- Zhang X., Xie C., Lin A.S., Ito H., Awad H., Lieberman J.R., Rubery P.T., Schwarz E.M., O'Keefe R.J., Guldberg R.E. Periosteal progenitor cell fate in segmental cortical bone graft transplantations: implications for functional tissue engineering. J. Bone Miner. Res. 2005;20(12):2124–2137. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

