Recent advances in endohedral metallofullerenes
- PMID: 40242547
- PMCID: PMC11997591
- DOI: 10.1016/j.fmre.2023.12.004
Recent advances in endohedral metallofullerenes
Abstract
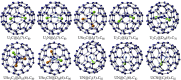
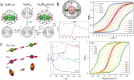
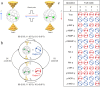
Fullerenes are a collection of closed polycyclic polymers consisting exclusively of carbon atoms. Recent remarkable advancements in the fabrication of metal-fullerene nanocatalysts and polymeric fullerene layers have significantly expanded the potential applications of fullerenes in various domains, including electrocatalysis, transistors, energy storage devices, and superconductors. Notably, the interior of fullerenes provides an optimal environment for stabilizing a diverse range of metal ions or clusters through electron transfer, resulting in the formation of a novel class of hybrid molecules referred to as endohedral metallofullerenes (EMFs). The utilization of advanced synthetic methodologies and the progress achieved in separation techniques have played a pivotal role in expanding the diversity of the encapsulated metal constituents, consequently leading to distinctive structural, electronic, and physicochemical properties of novel EMFs that surpass conventional ones. Intriguing phenomena, including regioselective dimerization between EMFs, direct metal-metal bonding, and non-classical cage preferences, have been unveiled, offering valuable insights into the coordination interactions between metallic species and carbon. Of particular importance, the recent achievements in the comprehensive characterization of EMFs based on transition metals and actinide metals have generated a particular interest in the exploration of new metal clusters possessing long-desired bonding features within the realm of coordination chemistry. These clusters exhibit a remarkable affinity for coordinating with non-metal atoms such as carbon, nitrogen, oxygen, and sulfur, thus making them highly intriguing subjects of systematic investigations focusing on their electronic structures and physicochemical properties, ultimately leading to a deeper comprehension of their unparalleled bonding characteristics. Moreover, the versatility conferred by the encapsulated species endows EMFs with multifunctional properties, thereby unveiling potential applications in various fields including biomedicine, single-molecule magnets, and electronic devices.
Keywords: Crystallography; Fullerenes; Metal-metal bonding; Metallofullerenes; Single-molecule magnet.
© 2023 The Authors. Publishing Services by Elsevier B.V. on behalf of KeAi Communications Co. Ltd.
Conflict of interest statement
The authors declare that they have no conflicts of interest in this work.
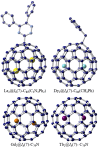
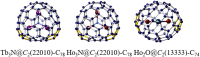
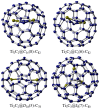
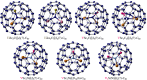
Figures


References
-
- Kroto H.W., Heath J.R., O'Brien S.C., et al. C60: Buckminsterfullerene. Nature. 1985;318(6042):162–163.
-
- Hirsch A., et al. The era of carbon allotropes. Nat. Mater. 2010;9:868–871. - PubMed
-
- Lu X., Feng L., Akasaka T., et al. Current status and future developments of endohedral metallofullerenes. Chem. Soc. Rev. 2012;41(23):7723–7760. - PubMed
-
- Yang S., Liu F., Chen C., et al. Fullerenes encaging metal clusters-clusterfullerenes. Chem. Commun. 2011;47(43):11822–11839. - PubMed
-
- Popov A.A., Yang S., Dunsch L., et al. Endohedral fullerenes. Chem. Rev. 2013;113(8):5989–6113. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

