Plant-derived nanovesicles: Promising therapeutics and drug delivery nanoplatforms for brain disorders
- PMID: 40242551
- PMCID: PMC11997602
- DOI: 10.1016/j.fmre.2023.09.007
Plant-derived nanovesicles: Promising therapeutics and drug delivery nanoplatforms for brain disorders
Abstract
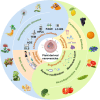
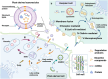
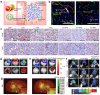
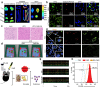
Plant-derived nanovesicles (PDNVs), including plant extracellular vesicles (EVs) and plant exosome-like nanovesicles (ELNs), are natural nano-sized membranous vesicles containing bioactive molecules. PDNVs consist of a bilayer of lipids that can effectively encapsulate hydrophilic and lipophilic drugs, improving drug stability and solubility as well as providing increased bioavailability, reduced systemic toxicity, and enhanced target accumulation. Bioengineering strategies can also be exploited to modify the PDNVs to achieve precise targeting, controlled drug release, and massive production. Meanwhile, they are capable of crossing the blood-brain barrier (BBB) to transport the cargo to the lesion sites without harboring human pathogens, making them excellent therapeutic agents and drug delivery nanoplatform candidates for brain diseases. Herein, this article provides an initial exposition on the fundamental characteristics of PDNVs, including biogenesis, uptake process, isolation, purification, characterization methods, and source. Additionally, it sheds light on the investigation of PDNVs' utilization in brain diseases while also presenting novel perspectives on the obstacles and clinical advancements associated with PDNVs.
Keywords: Brain disorders; Drug delivery platforms; Edible plants; Plant-derived nanovesicles; Traditional Chinese medicine.
© 2023 The Authors. Publishing Services by Elsevier B.V. on behalf of KeAi Communications Co. Ltd.
Conflict of interest statement
The authors declare that they have no conflicts of interest in this work.
Figures




References
-
- Halperin W., Jensen W.A. Ultrastructural changes during growth and embryogenesis in carrot cell cultures. J. Ultrastruct. Res. 1967;18(3):428–443. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

