Real-World Experience of Bimekizumab in a Cohort of 109 Patients Over 48 Weeks and Identification of Predictive Factors for an Early Super Response and Risk of Adverse Events
- PMID: 40242569
- PMCID: PMC12000911
- DOI: 10.2147/PTT.S514249
Real-World Experience of Bimekizumab in a Cohort of 109 Patients Over 48 Weeks and Identification of Predictive Factors for an Early Super Response and Risk of Adverse Events
Abstract
Introduction: Psoriasis is a chronic inflammatory skin disease significantly impairing quality of life. The introduction of biologic therapies, such as bimekizumab-a monoclonal antibody targeting IL-17A and IL-17F-has revolutionized treatment outcomes. This study investigates the effectiveness of bimekizumab in a real-world setting, focusing on the predictors of Early Super Response (ESR), defined as achieving PASI 100 by week 4, and evaluates the safety profile over a 48-week follow-up period.
Methods: A retrospective study was conducted on 109 psoriasis patients treated with bimekizumab at two Italian dermatology centers. Of these, 61 patients completed a 48-weeks follow-up. Baseline clinical and demographic data, PASI scores at multiple time points, and adverse events were collected. ESR predictors were analyzed using univariate and multivariate logistic regression models. Safety was assessed using Cox proportional hazards models to find predictive factors associated with the risk of adverse events (AEs).
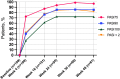
Results: At week 4, 28.4% of patients achieved PASI 100. Baseline PASI (OR: 0.93, p = 0.029), absence of nail involvement (OR: 0.12, p = 0.003), and fewer biologic failures (OR: 0.14, p = 0.038) were independently associated with ESR status. Safety analysis revealed that 15.6% of patients experienced adverse events, with asthma/allergic rhinitis significantly associated with a higher risk (HR: 6.43, p = 0.012). Candidiasis (7.3%) and eczema (4.6%) were the most common adverse events.
Conclusion: Bimekizumab demonstrated significant effectiveness and an acceptable safety profile in a real-world setting. Baseline PASI, nail involvement, and prior biologic failures influenced early treatment response. Identifying predictors of ESR and adverse events can guide personalized therapeutic approaches, optimizing outcomes for psoriasis patients.
Keywords: asthma; bimekizumab; efficacy; nails; safety; super responder.
© 2025 Fratton et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures
References
LinkOut - more resources
Full Text Sources
Miscellaneous