Persistent Trichomoniasis in Pregnancy: A Case Report Calling for Further Research to Alternative Antibiotic Treatment
- PMID: 40242693
- PMCID: PMC12001296
- DOI: 10.7759/cureus.80702
Persistent Trichomoniasis in Pregnancy: A Case Report Calling for Further Research to Alternative Antibiotic Treatment
Abstract
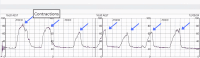
Trichomoniasis is an STI caused by a microscopic parasite known as Trichomonas vaginalis. It is considered one of the most widespread nonviral STDs globally. Trichomoniasis during pregnancy can lead to complications such as early rupture of membranes and premature birth. The standard treatment for trichomoniasis in adults, including pregnant individuals, is metronidazole. Recurrent cases may occur due to factors such as incomplete treatment, reinfection, or metronidazole resistance. In cases of persistent trichomoniasis where noncompliance and reinfection have been ruled out, metronidazole resistance should be considered. We present the case of a 39-year-old woman, gravida 4, para 3, with persistent trichomoniasis unresponsive to multiple courses of metronidazole, suggesting potential metronidazole resistance. She experienced recurrent symptoms of threatened preterm labor, likely due to uterine irritability caused by trichomoniasis. While various treatment regimens are available for metronidazole resistance, their safety profiles have not been evaluated during pregnancy. Further research is essential to identify a safe and effective treatment for metronidazole-resistant trichomoniasis in pregnancy.
Keywords: metronidazole resistance; metronidazole-resistant trichomoniasis; persistent trichomoniasis; preterm birth; recurrent trichomoniasis infection; sexually transmitted diseases; threatened preterm labor; trichomonas vaginalis; trichomoniasis; trichomoniasis in pregnancy.
Copyright © 2025, Sabet et al.
Conflict of interest statement
Human subjects: Consent for treatment and open access publication was obtained or waived by all participants in this study. Human Research Ethics Committee issued approval Ex64-24_113921. This project was reviewed by the Human Research Ethics Committee Chair and approved for exemption for ethics approval. It was deemed compliant with the NHMRC guidance “Ethical Consideration in Quality Assurance and Evaluation Activities” 2014. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources