Rate of fusion using novel synthetic bone graft mixed with cellular allograft product in lumbar fusions
- PMID: 40242813
- PMCID: PMC11998053
- DOI: 10.21037/jss-24-87
Rate of fusion using novel synthetic bone graft mixed with cellular allograft product in lumbar fusions
Abstract
Background: Over 400,000 spine fusions are performed in the United States annually with 75% involving the lumbar region. It is the indicated treatment of many chronic orthopedic conditions that fail conservative management. There are numerous surgical approaches; however, common to all is the removal of the intervertebral disc and the insertion of a bone graft which promotes arthrodesis. Iliac crest autografts are regarded as the "gold standard" bone graft material for lumbar fusions; however, they come with a significant complication rate. Recently developed biologic mixtures, such as the one used in this study, have illustrated similar qualities to autograft material. This study aims to observe how the mixture of a cellular allograft with a fully synthetic bone graft will affect the rate of arthrodesis in patients undergoing lumbar fusions.
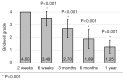
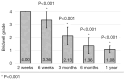
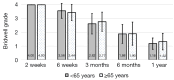
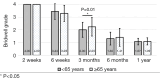
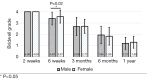
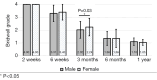
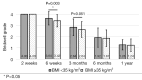
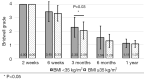
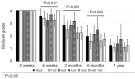
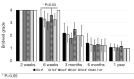
Methods: A retrospective chart review on patients who received a lumbar interbody fusion using a combination of Vimax® and Osteoflo® between May 26, 2021, to December 31, 2022, was performed. Demographic information was obtained. Pre-operative radiographs were measured in addition to 2-week, 6-week, 3-month, 6-month, and 1-year post-operative radiographs. Post-operative radiographs were examined to assign a Bridwell fusion grade to interbody and lateral mass fusions.
Results: A total of 129 patients receiving 211 lateral mass fusions and 199 interbody fusions were studied. A proportion of 3.3% of lateral mass fusions recorded a Bridwell Grade I (complete fusion) at 3 months post-operative and 77.8% at 1 year post-operative. Among interbody fusions, 14.1% were assigned a Bridwell Grade I at 3-month post-operative and 92.0% at the 1-year post-operative timepoint. Non-modifiable risk factors such as age and sex at birth had no impact on arthrodesis rate at 1 year for lateral mass or interbody fusions. Additionally, there was no significant difference in long-term fusions rates at the 1-year post-operative mark between obese and non-obese groups. Comorbidities did not affect the rate of arthrodesis 1-year post-operative apart from depression and hypertension. Patients with depression, and those without hypertension, exhibited significantly reduced lateral mass fusion rates with no difference in interbody fusion rates. While significant variations in rates of fusion were noted amongst surgical approaches at intermediate time points, no difference was observed 1 year post-operatively. Significant improvements in spondylolisthesis, anterior disc height, posterior disc height, and foraminal height were observed at each post-operative period.
Conclusions: The cellular allograft and synthetic mixture demonstrated significant arthrodesis rate at 92%, which trends higher than historically reported results for iliac crest autograft. Important to note, the absence of reduced arthrodesis rate in particular at-risk groups, such as the elderly, obese, and those with osteoporosis, suggests this mixture can be used in an extensive patient population and can overcome historically challenging arthrodesis patient cohorts.
Keywords: Lumbar interbody fusion; arthrodesis rate; cellular allograft; synthetic bone graft.
Copyright © 2025 AME Publishing Company. All rights reserved.
Conflict of interest statement
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://jss.amegroups.com/article/view/10.21037/jss-24-87/coif). S.B. has no funding from SurGenTec, Indiana Clinical and Translational Sciences Institute, and the National Institute of Health (No. UL1TR002529). M.S. reports relationships with Ventris Medical (consultant) and SurGenTec (consultant, royalties, and stock options). The authors have no other conflicts of interest to declare.
Figures


Similar articles
-
Successful Arthrodesis Using a Blended Allograft and Autograft Mixture in Lumbar Interbody Fusion: A Retrospective Case Series.Cureus. 2024 Sep 15;16(9):e69476. doi: 10.7759/cureus.69476. eCollection 2024 Sep. Cureus. 2024. PMID: 39416547 Free PMC article.
-
Posterior lumbar interbody fusion using recombinant human bone morphogenetic protein type 2 with cylindrical interbody cages.Spine J. 2004 Sep-Oct;4(5):527-38; discussion 538-9. doi: 10.1016/j.spinee.2004.03.025. Spine J. 2004. PMID: 15363423 Clinical Trial.
-
Healos and bone marrow aspirate used for lumbar spine fusion: a case controlled study comparing healos with autograft.Spine (Phila Pa 1976). 2006 Aug 15;31(18):E636-40. doi: 10.1097/01.brs.0000232028.97590.12. Spine (Phila Pa 1976). 2006. PMID: 16915079 Clinical Trial.
-
Randomized clinical trial: expanded autologous bone marrow mesenchymal cells combined with allogeneic bone tissue, compared with autologous iliac crest graft in lumbar fusion surgery.Spine J. 2020 Dec;20(12):1899-1910. doi: 10.1016/j.spinee.2020.07.014. Epub 2020 Jul 28. Spine J. 2020. PMID: 32730985 Clinical Trial.
-
Interbody cages versus structural bone grafts in lumbar arthrodesis: a systematic review and meta-analysis.J Neurosurg Spine. 2024 May 10;41(2):188-198. doi: 10.3171/2024.2.SPINE23940. Print 2024 Aug 1. J Neurosurg Spine. 2024. PMID: 38728766
References
LinkOut - more resources
Full Text Sources
