O-Trifluoromethylation of ketones: an alternative straightforward route to alkenyl trifluoromethyl ethers
- PMID: 40242851
- PMCID: PMC11997862
- DOI: 10.1039/d5sc01073j
O-Trifluoromethylation of ketones: an alternative straightforward route to alkenyl trifluoromethyl ethers
Abstract
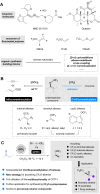
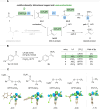
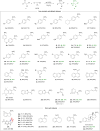
Here we report an unprecedented O-trifluoromethylation of ketones using chloro(phenyl)trifluoromethyl-λ3-iodane (CPTFI). Our method provides a new strategy for the facile synthesis of various synthetically valuable alkenyl trifluoromethyl ethers, particularly those CF3O-substituted terminal alkenes and cyclic alkenes that have been elusive until now, from simple aromatic, aliphatic, and cyclic ketones. The success of this reaction is attributed to the full utilization of the multifunctionality of CPTFI: (1) its strong Lewis acid activation ability, which enables weak nucleophiles such as Cl anions to attack the carbonyl group; (2) its bifunctionality, which allows for the introduction of CF3 and Cl into the carbonyl group in one step, thus enabling the obtainment of alkenyl trifluoromethyl ethers by further removal of HCl. The further transformation in the synthesis of CF3O-cyclopropanes, which were previously largely unexplored, reveals the significant potential of alkenyl trifluoromethyl ethers as valuable CF3O-containing building blocks in the discovery of innovative materials, pharmaceuticals, and agrochemicals.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


Similar articles
-
Direct C-H trifluoromethylation of di- and trisubstituted alkenes by photoredox catalysis.Beilstein J Org Chem. 2014 May 12;10:1099-106. doi: 10.3762/bjoc.10.108. eCollection 2014. Beilstein J Org Chem. 2014. PMID: 24991259 Free PMC article.
-
General Synthesis of N-Trifluoromethyl Compounds with N-Trifluoromethyl Hydroxylamine Reagents.J Am Chem Soc. 2022 Feb 2;144(4):1962-1970. doi: 10.1021/jacs.1c12467. Epub 2022 Jan 19. J Am Chem Soc. 2022. PMID: 35045700
-
Silver-Mediated Oxidative Trifluoromethylation of Phenols: Direct Synthesis of Aryl Trifluoromethyl Ethers.Angew Chem Int Ed Engl. 2015 Sep 28;54(40):11839-42. doi: 10.1002/anie.201506329. Epub 2015 Aug 12. Angew Chem Int Ed Engl. 2015. PMID: 26268604
-
Oxidative trifluoromethylation and trifluoromethylthiolation reactions using (trifluoromethyl)trimethylsilane as a nucleophilic CF3 source.Acc Chem Res. 2014 May 20;47(5):1513-22. doi: 10.1021/ar4003202. Epub 2014 Apr 28. Acc Chem Res. 2014. PMID: 24773518 Review.
-
Recent advances in trifluoromethylation of organic compounds using Umemoto's reagents.Org Biomol Chem. 2014 Sep 14;12(34):6580-9. doi: 10.1039/c4ob00671b. Org Biomol Chem. 2014. PMID: 25011917 Review.
References
-
- Charpentier J. Früh N. Togni A. Chem. Rev. 2015;115:650. doi: 10.1021/cr500223h. - DOI - PubMed
- Alonso C. de Marigorta E. M. Rubiales G. Palacios F. Chem. Rev. 2015;115:1847. doi: 10.1021/cr500368h. - DOI - PubMed
- Liu X. Xu C. Wang M. Liu Q. Chem. Rev. 2015;115:683. - PubMed
- Liang T. Neumann C. N. Ritter T. Angew. Chem., Int. Ed. 2013;52:8214. doi: 10.1002/anie.201206566. - DOI - PubMed
-
-
CF3O-related reviews:
- Hao B.-Y. Han Y.-P. Zhang Y.-C. Liang Y.-M. Org. Biomol. Chem. 2023;21:4926. doi: 10.1039/D3OB00258F. - DOI - PubMed
- Si Y.-F. Tang P.-P. Chin. J. Chem. 2023;41:2179.
- Tlili A. Toulgoat F. Billard T. Angew. Chem., Int. Ed. 2016;55:11726. - PubMed
- Besset T. Jubault P. Pannecoucke X. Poisson T. Org. Chem. Front. 2016;3:1004. doi: 10.1039/C6QO00164E. - DOI
-
-
- Liu J. Lin W.-K. Sorochinsky A. E. Greg Butler G. Landa A. Han J.-L. Soloshonok V. A. J. Fluorine Chem. 2022:257.
-
and references therein;
- Inoue M. Sumii Y. Shibata N. ACS Omega. 2020;5:10633. doi: 10.1021/acsomega.0c00830. - DOI - PMC - PubMed
- Ogawa Y. Tokunaga E. Kobayashi O. Hirai K. Shibata N. iScience. 2020;23:101467. doi: 10.1016/j.isci.2020.101467. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

