Blood-Brain Barrier Leakage in the Penumbra Is Associated With Infarction on Follow-Up Imaging in Acute Ischemic Stroke
- PMID: 40242873
- PMCID: PMC12180707
- DOI: 10.1161/STROKEAHA.124.050171
Blood-Brain Barrier Leakage in the Penumbra Is Associated With Infarction on Follow-Up Imaging in Acute Ischemic Stroke
Abstract
Background: Blood-brain barrier (BBB) leakage measured with dynamic susceptibility contrast-enhanced magnetic resonance imaging (MRI) has been associated with hemorrhagic transformation in acute ischemic stroke. However, the influence of prethrombolysis BBB leakage on infarct growth has not been studied. Therefore, we aimed to characterize BBB integrity according to tissue state at admission and tissue fate on follow-up MRI.
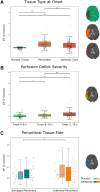
Methods: This is a post hoc analysis of the WAKE-UP trial (Efficacy and Safety of MRI-Based Thrombolysis in Wake-Up Stroke). Ischemic cores were segmented on diffusion-weighted imaging at baseline and on fluid-attenuated inversion recovery images at follow-up (22-36 hours). Dynamic susceptibility contrast-enhanced-MRI provided penumbra masks (time to maximum of the tissue residue function >6 s minus ischemic core) and BBB leakage (extraction fraction [EF], Z scored) maps via automated analysis. EF was averaged within the ischemic core, total penumbra, 2 penumbra subtypes (salvaged/infarcted penumbra), and normal tissue. Adjusted linear mixed-effects models tested for differences between tissue types and associations of EF with clinical/imaging outcomes. Complementary voxel-wise analyses were performed.
Results: Of 503 patients enrolled in the trial, 165 with suitable dynamic susceptibility contrast-enhanced-MRI data were included in this analysis (mean age 66 years, 38% women, median National Institutes of Health Stroke Scale score of 6; 53% receiving alteplase). EF was significantly increased in the ischemic core and penumbra relative to normally perfused tissue, while differences between total penumbra and ischemic core were statistically nonsignificant. Infarcted penumbra exhibited higher EF than salvaged penumbra, even after adjusting for hypoperfusion severity (P<0.001, n=79 with baseline penumbral tissue and follow-up MRI). Voxel-wise analyses showed a significant association between EF and voxel-level infarction in the placebo group only. EF did not predict hemorrhagic transformation or functional outcomes.
Conclusions: Penumbral BBB leakage may identify tissue at increased risk of infarction. Larger, prospective studies are needed to determine the clinical relevance of BBB leakage as an imaging marker of tissue fate.
Registration: URL: https://www.clinicaltrials.gov; Unique identifier: NCT01525290. URL: https://eudract.ema.europa.eu/; Unique identifier: 2011-005906-32.
Keywords: blood-brain barrier; cerebral hemorrhage; infarction; ischemic stroke; magnetic resonance imaging; plasminogen activators.
Conflict of interest statement
Dr Schlemm reports grants from Gemeinnützige Hertie-Stiftung. Dr Fiehler reports compensation from Stryker Corporation, TG Medical, Tonbridge, and Roche for consultant services. Dr Muir reports compensation from Woolsey Pharma, AbbVie, and Biogen for consultant services. Dr Thijs reports employment by Austin Health; compensation from Medtronic, Bayer, Boehringer Ingelheim, and Amgen, for consultant services; compensation from American Heart Association and AtriCure Inc for other services. Dr Thomalla reports grants from FP7 Health, European Union, and Deutsche Forschungsgemeinschaft and compensation from AstraZeneca for consultant services. The other authors report no conflicts.
Figures

References
-
- Thomalla G, Gerloff C. Acute imaging for evidence-based treatment of ischemic stroke. Curr Opin Neurol. 2019;32:521–529. doi: 10.1097/WCO.0000000000000716 - PubMed
-
- Thomalla G, Simonsen CZ, Boutitie F, Andersen G, Berthezene Y, Cheng B, Cheripelli B, Cho T-H, Fazekas F, Fiehler J, et al. ; WAKE-UP Investigators. MRI-guided thrombolysis for stroke with unknown time of onset. N Engl J Med. 2018;379:611–622. doi: 10.1056/NEJMoa1804355 - PubMed
-
- Astrup J, Siesjö BK, Symon L. Thresholds in cerebral ischemia—the ischemic penumbra. Stroke. 1981;12:723–725. doi: 10.1161/01.str.12.6.723 - PubMed
-
- Jovin TG, Nogueira RG, Lansberg MG, Demchuk AM, Martins SO, Mocco J, Ribo M, Jadhav AP, Ortega-Gutierrez S, Hill MD, et al. Thrombectomy for anterior circulation stroke beyond 6 h from time last known well (AURORA): a systematic review and individual patient data meta-analysis. Lancet. 2022;399:249–258. doi: 10.1016/S0140-6736(21)01341-6 - PubMed
-
- Pensato U, Lun R, Demchuk A. Thrombectomy in medium to large ischemic core: do patients still need to be SELECTed? JAMA. 2024;331:736–738. doi: 10.1001/jama.2023.27154 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

