Methodological limitations and confounders in dermal toxicity evaluation of aqueous test substance by OECD technical guidelines 402, 410: our experience of testing ethanol based hand sanitizer
- PMID: 40242887
- PMCID: PMC12006945
- DOI: 10.1080/07853890.2025.2491664
Methodological limitations and confounders in dermal toxicity evaluation of aqueous test substance by OECD technical guidelines 402, 410: our experience of testing ethanol based hand sanitizer
Abstract
Background: In view of overzealous use of Alcohol based hand sanitizer (ABHS) during COVID-19 pandemic and associated alarming rise in the cases of hand eczema and dermatitis around the world. We conducted an in vivo dermal toxicity with objective of exploring the acute and subacute effects of ethanol based hand sanitizer (EBHS) on Sprague Dawley rats.
Aims: To evaluate acute and subacute dermal toxicity due to ethanol based hand sanitizer (EBHS) on Sprague Dawley rats.
Methods: In first phase, following Organisation for Economic Co-operation and Development (OECD) Technical Guidelines (TG) 402, we conducted acute dermal toxicity study with two rats and EBHS containing 72.34% ethanol. In second phase, sub-acute dermal toxicity study was conducted, following OECD TG 410 with five groups of rats (10 animals of either sexes in each group) at various doses.
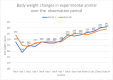
Results: In both the studies, no erythema, oedema, and eschar was observed. Although no clinical signs of toxicity were observed in both the studies, one death was encountered in subacute study. Macroscopically skin was normal; however, microscopic changes such as hyperkeratosis, parakeratosis, erosion, and extracellular oedema in epidermis and diffuse inflammatory cell infiltration in dermis was observed, suggestive of spongiotic dermatitis and 'clinic-pathological discordance'. However, attributing this changes to ethanol is difficult due to methodological limitations and confounders.
Conclusion: In both the studies, ethanol based hand sanitizer (EBHS) was found to be non-irritant with LD50 of > 2000 mg/kg and classified as Class 5/Unclassified according to GHS classification. Although, spongiotic changes were observed, methodological limitation of absence of control group in TG 402 and confounding effect of water and occlusion in all the animals/groups in both studies prevented us to attribute it to ethanol.
Keywords: OECD TG 402; OECD TG 410; acute dermal toxicity; ethanol based hand sanitizer; subacute dermal toxicity.
Plain language summary
In COVID19 pandemic, an emergency measure of hand hygiene, i.e. Alcohol -based hand sanitizers (ABHS); Ethanol- based hand sanitizer (EBHS) in Indian context, has been used overzealously for prolonged period. This has led to rise in cases of skin changes in hands, which was reported by many dermatologists around the world. We tested a commonly used EBHS by applying it to rat skin on a single dose in acute dermal toxicity evaluation and repeated dose in subacute dermal toxicity evaluation. Although, we did not find gross skin changes in both the studies, microscopic changes in the epidermis and dermis suggestive of spongiotic dermatitis were observed. However, due to absence of control group in acute study, and presence of water in test substance along with occlusion of skin which independently can give rise to such microscopic picture, we could not implicate ethanol for microscopic changes in skin. Such situation demands modification in methodology in OECD TG 402 and 410 while testing an aqueous test substance. Also, simultaneous conduct of both acute and subacute studies is recommended for testing aqueous test substance because acute study conducted in isolation without control group may give erroneous results. In view of such inbuilt methodological limitations and confounder, use of newer in vitro, and molecular techniques is recommended for reliable toxicological evaluation of aqueous base substance.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures



Similar articles
-
COVID-19, Overzealous Sanitizer Use, and Hair Discoloration: Case Reports.Acta Dermatovenerol Croat. 2022 Jul;30(1):57-58. Acta Dermatovenerol Croat. 2022. PMID: 36153721
-
Biometrical evaluation of the performance of the revised OECD Test Guideline 402 for assessing acute dermal toxicity.Regul Toxicol Pharmacol. 2017 Oct;89:26-39. doi: 10.1016/j.yrtph.2017.07.007. Epub 2017 Jul 11. Regul Toxicol Pharmacol. 2017. PMID: 28709685
-
Serious Adverse Health Events, Including Death, Associated with Ingesting Alcohol-Based Hand Sanitizers Containing Methanol - Arizona and New Mexico, May-June 2020.MMWR Morb Mortal Wkly Rep. 2020 Aug 14;69(32):1070-1073. doi: 10.15585/mmwr.mm6932e1. MMWR Morb Mortal Wkly Rep. 2020. PMID: 32790662 Free PMC article.
-
Toxicity of chemical-based hand sanitizers on children and the development of natural alternatives: a computational approach.Crit Rev Toxicol. 2023 Dec;53(9):572-599. doi: 10.1080/10408444.2023.2270496. Epub 2023 Dec 5. Crit Rev Toxicol. 2023. PMID: 37916473 Review.
-
Final report on the safety assessment of capsicum annuum extract, capsicum annuum fruit extract, capsicum annuum resin, capsicum annuum fruit powder, capsicum frutescens fruit, capsicum frutescens fruit extract, capsicum frutescens resin, and capsaicin.Int J Toxicol. 2007;26 Suppl 1:3-106. doi: 10.1080/10915810601163939. Int J Toxicol. 2007. PMID: 17365137 Review.
References
-
- World Health Organization (WHO) . www.who.int (n.d.). [retrieved 2023 September 13]. Available from: https://apps.who.int/iris/handle/10665/331846
-
- Ministry of Consumer Affairs, Food & Public Distribution, Department of Food & Public Distribution, Government of India, Vol. 1. (2020). 2020-SP-l [review of No. 1(2)2020-SP-l]. Dfpd.gov.in. Available from: https://dfpd.gov.in/LwB3AHIAaQB0AGUAcgBlAGEAZABkAGEAdABhAC8AUABvAHIAdABh...
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
