BCL2A1‑ and G0S2‑driven neutrophil extracellular traps: A protective mechanism linking preeclampsia to reduced breast cancer risk
- PMID: 40242964
- PMCID: PMC12030921
- DOI: 10.3892/or.2025.8897
BCL2A1‑ and G0S2‑driven neutrophil extracellular traps: A protective mechanism linking preeclampsia to reduced breast cancer risk
Abstract
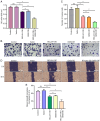
Preeclampsia has been associated with a reduced risk of breast cancer (BC), but the mechanisms underlying this relationship remain unclear. It has been suggested that neutrophil extracellular traps (NETs), which are released upon neutrophil activation, play a key role in both preeclampsia and BC. To investigate this link, the single‑cell RNA sequencing dataset GSE173193 was analyzed and upregulated genes BCL2A1 and G0/G1 switch gene 2 (G0S2) were identified in neutrophils from preeclamptic placentas. These findings were validated using reverse transcription‑quantitative PCR and western blotting. Combined analyses of preeclampsia and BC tissues, from Gene Expression Omnibus (GSE24129) and The Cancer Genome Atlas databases respectively, identified 2,040 upregulated differentially expressed genes, including BCL2A1 and G0S2. Furthermore, these genes showed clinical relevance to BC, as demonstrated by Receiver Operating Characteristic curve, survival analyses and weighted gene co‑expression network analysis. Functional experiments revealed that overexpression of BCL2A1 and G0S2 increased NET release and inhibited BC cell proliferation, invasion and migration. The present study provides novel insights into the shared molecular pathways of preeclampsia and BC, emphasizing NETs as a potential protective mechanism as increased NET production in preeclampsia may contribute to a reduced BC risk by influencing tumor progression and offer avenues for further research into therapeutic interventions.
Keywords: BCL2A1; G0S2; NETs; breast cancer; neutrophils; preeclampsia.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures









References
-
- Piani F, Agnoletti D, Baracchi A, Scarduelli S, Verde C, Tossetta G, Montaguti E, Simonazzi G, Degli Esposti D, Borghi C, HDP Bologna Study Group Serum uric acid to creatinine ratio and risk of preeclampsia and adverse pregnancy outcomes. J Hypertens. 2023;41:1333–1338. doi: 10.1097/HJH.0000000000003472. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

