Discriminating evidence - use and misuse of the drug-discrimination test in abuse potential assessment of novel CNS drugs
- PMID: 40243002
- PMCID: PMC12267871
- DOI: 10.1177/02698811251330780
Discriminating evidence - use and misuse of the drug-discrimination test in abuse potential assessment of novel CNS drugs
Abstract
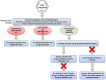
Nonclinical testing to predict the abuse potential of central nervous system (CNS) drug candidates is a mandatory part of the safety pharmacology assessment for medications seeking approval for human use. In the "standard model," the drug candidate is tested to determine whether its psychoactive effects generalize to the discriminative cue of an abused drug that animals have been trained to recognize. However, CNS drugs with novel pharmacological mechanisms are challenging, and in response, the regulatory agencies have recommended alternative experimental designs. Variant 1: test the drug candidate in a series of drug-discrimination experiments that exemplify the major classes of abused drugs. Variant 2: use the drug candidate as a training cue. Back-test examples from established classes of abused drugs to see if they generalize to the drug candidate's cue. We critically assessed the pharmacological and translational validity of these protocols. The standard model is underpinned by decades of research and refinement and has the highest degree of translational validity. Question marks exist over the validity of substitution results when the drug candidate has no affinity for known abuse-related targets. Published research does not support the use of either of the alternative models. On the contrary, these models have no pharmacological rationale and, consequently, no translational validity. The review contains a decision tree on the appropriate application of the standard drug-discrimination model, together with recommendations for adapting the test when characterizing the psychoactive properties of drug candidates acting on novel CNS targets.
Keywords: CNS drugs; Drug discrimination; methodology; predictive validity; rats; translational validity.
Conflict of interest statement
Declaration of conflicting interestsThe author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Heal DJ, Gosden J, and Smith SL are shareholders and employees of DevelRx Ltd. DevelRx provides consultancy to a wide range of pharmaceutical companies. Rowlett JK is Professor and Vice Chair for research in the Department of Psychiatry and Human Behavior at the University of Mississippi Medical Center (UMMC). He is a co-founder of the Center for Innovation and Discovery in Addictions (CIDA), a multidisciplinary group developing and employing novel treatments for addiction.
Figures




References
-
- Arnt J, Hyttel J. (1990) Dopamine D-2 agonists with high and low efficacies: Differentiation by behavioural techniques. J Neural Transm Gen Sect 80: 33–50. - PubMed
-
- Ator NA. (1999) High-dose discrimination training with midazolam: Context determines generalization profile. Pharmacol Biochem Behav 64: 237–243. - PubMed
-
- Ator NA, Grant KA, Purdy RH, et al. (1993) Drug discrimination analysis of endogenous neuroactive steroids in rats. Eur J Pharmacol 241: 237–243. - PubMed
-
- Ator NA, Griffiths RR. (1983) Lorazepam and pentobarbital drug discrimination in baboons: Cross-drug generalization and interaction with Ro 15-1788. J Pharmacol Exp Ther 226: 776–782. - PubMed
-
- Ator NA, Griffiths RR. (1985) Lorazepam and pentobarbital discrimination: Interactions with CGS 8216 and caffeine. Eur J Pharmacol 107: 169–181. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

