Protocol MelatoSom-Kids-PTSD: sleep disturbances in children and adolescents with post-traumatic stress disorder (PTSD) - a randomized double-blind placebo-controlled trial to investigate the efficacy of paediatric prolonged-release melatonin
- PMID: 40243149
- PMCID: PMC12006942
- DOI: 10.1080/20008066.2025.2474375
Protocol MelatoSom-Kids-PTSD: sleep disturbances in children and adolescents with post-traumatic stress disorder (PTSD) - a randomized double-blind placebo-controlled trial to investigate the efficacy of paediatric prolonged-release melatonin
Abstract
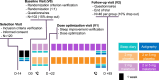
Introduction: Sleep disorders in the insomnia spectrum, as well as nightmares, are among the most sensitive and persistent symptoms in children with post-traumatic stress disorder (PTSD). There is currently no reference treatment or specific pharmacological treatment recommendation on the management of sleep disturbances in children and adolescents suffering from PTSD, despite the fact that they have a significant effect on daytime functioning and overall mental health of children as well as on family's health and quality of life. In this respect, paediatric prolonged-release melatonin (PedPRM) has shown significant beneficial effects on insomnia disorders in children with autism spectrum disorders and positive effects on anxiety and depressive symptomatology. Our study will be the first randomized controlled trial to examine the efficacy of PedPRM melatonin on sleep disorders in children and adolescents with PTSD, as well as on PTSD symptoms, associated daytime functioning and overall mental health in these children and their caregivers.Methods/design: The MelatoSOM-Kids-PTSD study (French national hospital-based clinical research programme) will be a multi-centre prospective double-blind placebo-controlled parallel group clinical trial investigating the efficacy of paediatric prolonged-release melatonin to alleviate sleep disturbances in children and adolescents with PTSD (120 participants recruited over a 24-month period). The experimental group will be treated with active prolonged-release melatonin over 13 weeks (PedPRM). The control group will receive a placebo. The primary endpoint will be the difference in sleep diary derived total sleep time after 13 weeks of treatment in the PedPRM group versus placebo group. Secondary endpoints will be the differences in objective sleep quality parameters and daytime functioning before and after treatment, in children with PTSD and their caregivers.Discussion: This paper describes the MelatoSOM-Kids-PTSD protocol, which will evaluate the effectiveness of melatonin, a treatment that has already demonstrated an excellent benefit-risk ratio in the paediatric population over 4 years.
Introducción: Los trastornos del sueño dentro del espectro del insomnio, así como las pesadillas, se encuentran entre los síntomas más sensibles y persistentes en niños con trastorno de estrés postraumático (TEPT). Actualmente, no existe un tratamiento de referencia ni una recomendación farmacológica específica para el manejo de las alteraciones del sueño en niños y adolescentes con TEPT, a pesar de que dichos trastornos influyen de forma significativa en el funcionamiento diurno y en la salud mental global de los menores, así como en la salud y calidad de vida de sus familias. En este sentido, la melatonina pediátrica de liberación prolongada (PedPRM, por sus siglas en inglés) ha mostrado efectos beneficiosos importantes en los trastornos de insomnio en niños con trastornos del espectro autista, además de efectos positivos sobre la sintomatología ansiosa y depresiva. Nuestro estudio será el primer ensayo clínico controlado aleatorizado que evalúe la eficacia de la PedPRM en los trastornos del sueño de niños y adolescentes con TEPT, así como en los síntomas propios del TEPT, el funcionamiento diurno asociado y la salud mental general de estos menores y sus cuidadores.
Método/Diseño: El estudio MelatoSom-Kids-PTSD (perteneciente a un programa francés de investigación clínica hospitalaria) será un ensayo clínico multicéntrico, prospectivo, doble ciego, controlado con placebo y con grupos paralelos, diseñado para investigar la eficacia de la melatonina pediátrica de liberación prolongada en el alivio de los trastornos del sueño en niños y adolescentes con TEPT (se reclutarán 120 participantes durante un período de 24 meses). El grupo experimental recibirá melatonina de liberación prolongada durante 13 semanas (PedPRM, según sus siglas en ingles). El grupo de control recibirá un placebo. El criterio de valoración principal será la diferencia en el tiempo total de sueño, calculado a partir del diario del sueño, tras 13 semanas de tratamiento en el grupo PedPRM frente al grupo placebo. Los criterios de valoración secundarios serán las diferencias en los parámetros objetivos de calidad del sueño y en el funcionamiento diurno antes y después del tratamiento, tanto en los niños con TEPT como en sus cuidadores.
Keywords: Melatonina de liberación prolongada; Sleep disorders; Trastorno de estrés postraumático; Trastornos del sueño; children; extended-release melatonin; niños; post-traumatic stress disorder; tratamiento; treatment.
Plain language summary
Unmet public health need with no specific pharmacological treatment recommended in the management of sleep disorders associated with childhood PTSD, despite these problems being prevalent and strongly associated with post-traumatic symptoms, deterioration in mental health, daytime functioning and quality of life in these children.Pioneering use of paediatric extended-release melatonin (PedPRM): The MelatSomPSD study is the first randomized controlled trial to evaluate the efficacy of PedPRM in children and adolescents with PTSD.Comprehensive outcome measures assessing not only the impact of PedPRM on sleep disorders but also its effects on PTSD symptoms, daytime functioning and overall mental health in children and their caregivers, ensuring an examination of both direct and indirect outcomes.
Conflict of interest statement
C. M. S. has been a study investigator of the multi-centre international PedPRM trials in children with ASD and Smith–Magenis syndrome and has received speaker honoraria by Neurim Pharmaceuticals, Biocodex and Medice. All other authors declare no conflict of interest related to this study.
Figures
References
-
- Alisic, E., van der Schoot, T. A. W., van Ginkel, J. R., & Kleber, R. J. (2008). Looking beyond posttraumatic stress disorder in children: Posttraumatic stress reactions, posttraumatic growth, and quality of life in a general population sample. The Journal of Clinical Psychiatry, 69(9), 1455–1461. 10.4088/jcp.v69n0913 - DOI - PubMed
-
- American Psychiatric Association . (2013). Diagnostic and statistical manual of mental disorders, fifth edition (DSM-5 (TM)). American Psychiatric Assoc. 10.1176/appi.books.9780890425596 - DOI
-
- Arendt, J., Bojkowski, C., Franey, C., Wright, J., & Marks, V. (1985). Immunoassay of 6-hydroxymelatonin sulfate in human plasma and urine: Abolition of the urinary 24-hour rhythm with atenolol. The Journal of Clinical Endocrinology & Metabolism, 60(6), 1166–1173. 10.1210/jcem-60-6-1166 - DOI - PubMed
-
- Beck, A. T., Steer, R. A., Ball, R., & Ranieri, W. (1996). Comparison of beck depression inventories -IA and -II in psychiatric outpatients. Journal of Personality Assessment, 67, 588–597. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical