Characterization of 2-phenanthroyl-CoA reductase, an ATP-independent type III aryl-CoA reductase involved in anaerobic phenanthrene degradation
- PMID: 40243319
- PMCID: PMC12093976
- DOI: 10.1128/aem.00166-25
Characterization of 2-phenanthroyl-CoA reductase, an ATP-independent type III aryl-CoA reductase involved in anaerobic phenanthrene degradation
Abstract
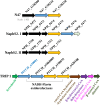
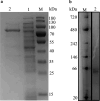
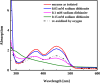
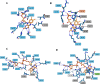
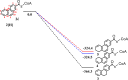
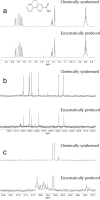
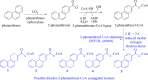
Anaerobic degradation of polycyclic aromatic hydrocarbons (PAHs) with three or more aromatic rings is extremely slow because the compounds are very poorly soluble in water and chemically stable. Phenanthrene is the only three-ring PAH where the anaerobic degradation has been partially elucidated. Phenanthrene is first activated via carboxylation producing 2-phenanthroate, which is further converted to 2-phenanthroyl-coenzyme A (CoA) via the enzyme 2-phenanthroate:CoA ligase. In this study, we elucidated the next degradation step, the reduction of 2-phenanthroyl-CoA to dihydro-2-phenanthroyl-CoA. We cloned the putative gene from the genome of culture TRIP_1 and heterologously expressed and purified the 2-phenanthroyl-CoA reductase enzyme from Escherichia coli. The identified monomeric flavo-enzyme belongs to the novel group of type III aryl-CoA reductases in the old-yellow enzyme family and has a molecular mass of 72 kDa. 2-Phenanthroyl-CoA reductase contains one FMN, one FAD, and one [4Fe-4S] iron-sulfur cluster as cofactors. The enzyme has a specific activity of 17.6 ± 0.4 nmol/min/mg, a Km value of 1.8 µM, and a Vmax of 7.9 µmol/min/mg at pH 7.5, when reduced methyl viologen was used as electron donor. 2-Phenanthroyl-CoA reductase catalyzed a two-electron reduction step producing one of five possible isomers. Quantum mechanical calculations and nuclear magnetic resonance analysis of the reaction product suggested 9,10-dihydro-2-phenanthroyl-CoA as the most stable isomer. However, our experimental evidence suggests 7,8-dihydro-2-phenanthroyl-CoA (International Union of Pure and Applied Chemistry [IUPAC]: 1,2-dihydro-7-phenanthroyl-CoA) or 5,6-dihydro-2-phenanthroyl-CoA (IUPAC: 3,4-dihydro-7-phenanthroyl-CoA) as the most likely reduced product with a saturated bond in ring 3 of the substrate 2-phenanthroyl-CoA, before undergoing isomerization changes to reach the more stable structure of 9,10-dihydro-2-phenanthroyl-CoA.IMPORTANCEPAHs are a group of highly toxic and persistent environmental pollutants. The anaerobic degradation of three-ring PAHs like phenanthrene is still poorly understood. Phenanthrene degradation starts with a carboxylation reaction to form 2-phenanthroic acid followed by a CoA-thioesterification reaction catalyzed by 2-phenanthroate:CoA ligase to produce 2-phenanthroyl-CoA. The next degradation step is the reduction of 2-phenanthroyl-CoA to dihydro-2-phenanthroyl-CoA to overcome the resonance energy of the aromatic ring system. Herein, we elucidated that the reduction reaction is catalyzed by the enzyme 2-phenanthroyl-CoA reductase. Furthermore, we provided biochemical and structural properties of the heterologously expressed and purified 2-phenanthroyl-CoA reductase, which confirmed that the enzyme belongs to the novel group of type III aryl-CoA reductases in the old-yellow enzyme family.
Keywords: 2-phenanthroyl-CoA; PAHs; anaerobic phenanthrene degradation; culture TRIP_1; old-yellow enzyme; reductase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

