Sex Differences in Maturation and Function of Neonatal Porcine Islets Upon Transplantation in Mice
- PMID: 40243327
- PMCID: PMC12005065
- DOI: 10.1111/xen.70039
Sex Differences in Maturation and Function of Neonatal Porcine Islets Upon Transplantation in Mice
Abstract
Background: Neonatal porcine islets (NPIs) can mature into a mixed population of endocrine cells that can restore glucose control in mice, pigs, and non-human primates, representing a potential alternative islet source for clinical beta cell replacement therapy. However, it remains unclear how conditions in the recipient influence the maturation and function of these cells. Here, we investigated the impact of host sex on NPIs implanted under the kidney capsule of male and female B6.129S7-Rag1tm1Mom (B6/Rag-/-) mice.
Methods: Diabetic mice were transplanted with 3000 NPIs under the kidney capsule. All mice were monitored for reversal of hyperglycemia and glucose clearance at 8- and 20-weeks post-transplant. Grafts were assessed for cell composition and insulin content.
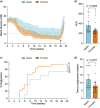
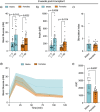
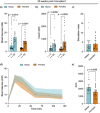
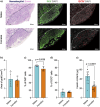
Results: Female mice demonstrated improved glucose clearance at 8- and 20-weeks post-transplant compared to their male counterparts. Improved glucose clearance correlated with accelerated diabetes reversal in females (8 weeks vs. 12 weeks in males) and increased rates of euglycemic achievement (17/18 in females vs. 14/19 in males). However, grafts collected from male mice exhibited an increased percentage of insulin-positive cells as well as increased insulin content.
Conclusion: The sex of the host influences the outcomes of NPI transplantation, showcasing the relevance of understanding the role of sex as a biological variable in islet transplantation.
Keywords: biological variables; diabetes; islet transplantation; neonatal porcine islets; sex differences; xenotransplantation.
© 2025 The Author(s). Xenotransplantation published by Wiley Periodicals LLC.
Figures

Similar articles
-
Functional Maturation and In Vitro Differentiation of Neonatal Porcine Islet Grafts.Transplantation. 2018 Oct;102(10):e413-e423. doi: 10.1097/TP.0000000000002354. Transplantation. 2018. PMID: 29975241
-
Cotransplantation of Mesenchymal Stem Cells With Neonatal Porcine Islets Improve Graft Function in Diabetic Mice.Diabetes. 2017 May;66(5):1312-1321. doi: 10.2337/db16-1068. Epub 2017 Feb 28. Diabetes. 2017. PMID: 28246290
-
Islet-derived damage-associated molecular pattern molecule contributes to immune responses following microencapsulated neonatal porcine islet xenotransplantation in mice.Xenotransplantation. 2016 Sep;23(5):393-404. doi: 10.1111/xen.12253. Epub 2016 Jul 15. Xenotransplantation. 2016. PMID: 27422454
-
Function, mass, and replication of porcine and rat islets transplanted into diabetic nude mice.Diabetes. 1995 Jan;44(1):104-11. doi: 10.2337/diab.44.1.104. Diabetes. 1995. PMID: 7813803
-
Islet xenotransplantation: what is the optimal age of the islet-source pig?Xenotransplantation. 2015 Jan-Feb;22(1):7-19. doi: 10.1111/xen.12130. Epub 2014 Aug 11. Xenotransplantation. 2015. PMID: 25130196 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

