Fructose activates a stress response shared by methylglyoxal and hydrogen peroxide in Streptococcus mutans
- PMID: 40243330
- PMCID: PMC12077213
- DOI: 10.1128/mbio.00485-25
Fructose activates a stress response shared by methylglyoxal and hydrogen peroxide in Streptococcus mutans
Abstract
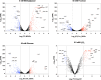
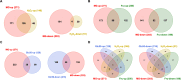
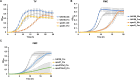
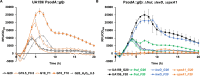
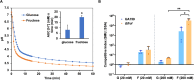
Fructose catabolism by Streptococcus mutans is initiated by three phosphotransferase (PTS) transporters yielding fructose-1-phosphate (F-1-P) or fructose-6-phosphate. Deletion of one such F-1-P-generating PTS, fruI, was shown to reduce the cariogenicity of S. mutans in rats fed a high-sucrose diet. Moreover, a recent study linked fructose metabolism in S. mutans to a reactive electrophile species methylglyoxal. Here, we conducted a comparative transcriptomic analysis of S. mutans treated briefly with 50 mM fructose, 50 mM glucose, 5 mM methylglyoxal, or 0.5 mM hydrogen peroxide (H2O2). The results revealed a striking overlap between the fructose and methylglyoxal transcriptomes, totaling 176 genes, 61 of which were also shared with the H2O2 transcriptome. This core of 61 genes encompassed many of the same pathways affected by exposure to low pH or zinc intoxication. Consistent with these findings, fructose negatively impacted the metal homeostasis of a mutant deficient in zinc expulsion and the growth of a mutant of the major oxidative stress regulator SpxA1. Importantly, fructose metabolism lowered culture pH at a faster pace, allowed better survival under acidic and nutrient-depleted conditions, and enhanced the competitiveness of S. mutans against Streptococcus sanguinis, although a moderated level of F-1-P might further boost some of these benefits. Conversely, several commensal streptococcal species displayed a greater sensitivity to fructose that may negatively affect their persistence and competitiveness in dental biofilm. In conclusion, fructose metabolism is integrated into the stress core of S. mutans and regulates critical functions required for survival and its ability to induce dysbiosis in the oral cavity.IMPORTANCEFructose is a common monosaccharide in the biosphere, yet its overconsumption has been linked to various health problems in humans including insulin resistance, obesity, diabetes, non-alcoholic liver diseases, and even cancer. These effects are in large part attributable to the unique biochemical characteristics and metabolic responses associated with the degradation of fructose. Yet, an understanding of the effects of fructose on the physiology of bacteria and its implications for the human microbiome is severely lacking. Here, we performed a series of analyses on the gene regulation of a dental pathogen Streptococcus mutans by exposing it to fructose and other important stress agents. Further supported by growth, persistence, and competition assays, our findings revealed the ability of fructose to activate a set of stress-related functions that may prove critical to the ability of the bacterium to persist and cause diseases both within and without the oral cavity.
Keywords: F-1-P; fructose; gene regulation; hydrogen peroxide; metal homeostasis; methylglyoxal; stress response.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Update of
-
FRUCTOSE ACTIVATES A STRESS RESPONSE SHARED BY METHYLGLYOXAL AND HYDROGEN PEROXIDE IN STREPTOCOCCUS MUTANS.bioRxiv [Preprint]. 2025 Mar 18:2024.10.26.620100. doi: 10.1101/2024.10.26.620100. bioRxiv. 2025. Update in: mBio. 2025 May 14;16(5):e0048525. doi: 10.1128/mbio.00485-25. PMID: 40166302 Free PMC article. Updated. Preprint.
Similar articles
-
FRUCTOSE ACTIVATES A STRESS RESPONSE SHARED BY METHYLGLYOXAL AND HYDROGEN PEROXIDE IN STREPTOCOCCUS MUTANS.bioRxiv [Preprint]. 2025 Mar 18:2024.10.26.620100. doi: 10.1101/2024.10.26.620100. bioRxiv. 2025. Update in: mBio. 2025 May 14;16(5):e0048525. doi: 10.1128/mbio.00485-25. PMID: 40166302 Free PMC article. Updated. Preprint.
-
Glycerol metabolism contributes to competition by oral streptococci through production of hydrogen peroxide.J Bacteriol. 2024 Sep 19;206(9):e0022724. doi: 10.1128/jb.00227-24. Epub 2024 Aug 22. J Bacteriol. 2024. PMID: 39171915 Free PMC article.
-
ActA-mediated PykF acetylation negatively regulates oxidative stress adaptability of Streptococcus mutans.mBio. 2024 Oct 16;15(10):e0183924. doi: 10.1128/mbio.01839-24. Epub 2024 Sep 9. mBio. 2024. PMID: 39248567 Free PMC article.
-
Preferred Hexoses Influence Long-Term Memory in and Induction of Lactose Catabolism by Streptococcus mutans.Appl Environ Microbiol. 2018 Jul 2;84(14):e00864-18. doi: 10.1128/AEM.00864-18. Print 2018 Jul 15. Appl Environ Microbiol. 2018. PMID: 29752268 Free PMC article.
-
Essential Roles of the sppRA Fructose-Phosphate Phosphohydrolase Operon in Carbohydrate Metabolism and Virulence Expression by Streptococcus mutans.J Bacteriol. 2018 Dec 20;201(2):e00586-18. doi: 10.1128/JB.00586-18. Print 2019 Jan 15. J Bacteriol. 2018. PMID: 30348833 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

