Protein-S-nitrosylation of human cytomegalovirus pp65 reduces its ability to undermine cGAS
- PMID: 40243337
- PMCID: PMC12090748
- DOI: 10.1128/jvi.00481-25
Protein-S-nitrosylation of human cytomegalovirus pp65 reduces its ability to undermine cGAS
Abstract
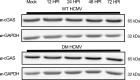
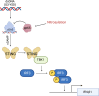
Post-translational modifications (PTMs) are key regulators of various processes important for cell survival. These modifications are critical for dealing with stress conditions, such as those observed in disease states, and during infections with various pathogens. We previously reported that during infection of primary dermal fibroblasts, multiple human cytomegalovirus (HCMV)-encoded proteins were post-translationally modified by the addition of a nitric oxide group to cysteine residues, a modification called protein-S-nitrosylation. For example, tegument protein pp71 is nitrosylated, diminishing its ability to inhibit STING, a protein necessary for DNA virus immune response. Herein, we report that an additional HCMV tegument protein, pp65, responsible for the inhibition of cGAS is also modified by protein-S-nitrosylation on two cysteine residues. Utilizing site-directed mutagenesis to generate recombinant viruses that encode a pp65 that cannot be protein-S-nitrosylated, we evaluated the impact of this PTM on viral replication and how the virus impacts the cGAS/STING pathway. We report that the nitrosylation of pp65 negatively impacts its ability to block cGAS enzymatic functions. pp65 protein-S-nitrosylation mutants demonstrated a decrease in cGAS/STING-induced IRF3 and TBK1 phosphorylation. Additionally, we observed a reduction in IFN-β1 secretion in NuFF-1 cells expressing a nitrosylation-resistant pp65. We report that HCMV expressing a protein-S-nitrosylation-deficient pp65 is resistant to the activation of cGAS in the infection of primary dermal fibroblasts. Our work suggests that nitrosylation of viral proteins may serve as a broadly neutralizing mechanism in HCMV infection.
Importance: Post-translational modifications (PTM) are utilized by host cells to limit an invading pathogen's ability to establish a productive infection. A potent PTM, called protein-S-nitrosylation, has anti-bacterial and anti-viral properties. Increasing protein-S-nitrosylation with the addition of nitric oxide donor compounds reduced HCMV replication in fibroblasts and epithelial cells. We previously reported that protein-S-nitrosylation of HCMV pp71 limits its ability to inhibit STING. Herein, we report that the protein-S-nitrosylation of HCMV pp65 impacts its ability to limit cGAS activity, an additional protein important in regulating interferon response. Therapeutically, patients provided nitric oxide by inhalation reduced viral replication in coronavirus disease 2019, influenza, and even impacted bacterial growth within patients' lungs. It is thought that an increase in free nitric oxide increases the frequency of nitrosylated proteins. Understanding how protein-S-nitrosylation regulates a common DNA virus like HCMV will provide insights into the development of broadly neutralizing therapeutics in drug-resistant viral infections.
Keywords: HCMV; PTM; STING; cGAS; herpesviruses; pp65; protein-S-nitrosylation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

