The Effect of Aldehyde and Carboxylic Acid Substitution on the Isomerization of Hemithioindigo Photoswitches
- PMID: 40243361
- PMCID: PMC12133626
- DOI: 10.1002/chem.202501108
The Effect of Aldehyde and Carboxylic Acid Substitution on the Isomerization of Hemithioindigo Photoswitches
Abstract
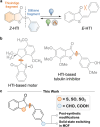
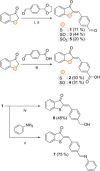
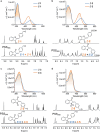
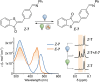
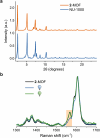
Hemithioindigo (HTI) photoswitches exhibit robust photoisomerization under visible light and relatively high thermal bistability. In this work, we report various modifications of the HTI core, namely the introduction of aldehydes and carboxylic acids at the para position of the stilbene fragment with different oxidation states of the sulfur center, and the incorporation of a Schiff base moiety. These modifications allowed tuning of the absorption properties, quantum yields of isomerization, and thermal stability of the metastable E-isomers. Notably, the formyl- and carboxyl-substituted HTI switches achieved high yields of isomerization under visible light in various solvents, while sulfur oxidation enhanced quantum yields but reduced photochromism. Schiff base formation led to red-shifted absorption and increased thermal stability. Finally, by leveraging the carboxyl substituents, we incorporated an HTI chromophore into the NU-1000 metal-organic framework (MOF), and demonstrated solid-state photoisomerization. These findings highlight key structural modifications that expand the applicability of HTI photoswitches for molecular switching in solution and solid-state environments.
Keywords: hemithioindigo; isomerization; metal‐organic frameworks; photochromism; photoswitches.
© 2025 The Author(s). Chemistry – A European Journal published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Making fast photoswitches faster--using Hammett analysis to understand the limit of donor-acceptor approaches for faster hemithioindigo photoswitches.Chemistry. 2014 Oct 20;20(43):13984-92. doi: 10.1002/chem.201403661. Epub 2014 Sep 11. Chemistry. 2014. PMID: 25214477
-
Indigoid Photoswitches: Visible Light Responsive Molecular Tools.Acc Chem Res. 2018 May 15;51(5):1153-1163. doi: 10.1021/acs.accounts.7b00638. Epub 2018 Apr 25. Acc Chem Res. 2018. PMID: 29694014
-
Oxidized Hemithioindigo Photoswitches-Influence of Oxidation State on (Photo)physical and Photochemical Properties.Chemistry. 2020 Aug 21;26(47):10712-10718. doi: 10.1002/chem.202002176. Epub 2020 Jul 23. Chemistry. 2020. PMID: 32485011 Free PMC article.
-
Light-Switchable Peptides with a Hemithioindigo Unit: Peptide Design, Photochromism, and Optical Spectroscopy.Chemphyschem. 2016 May 4;17(9):1252-63. doi: 10.1002/cphc.201501050. Epub 2016 Feb 4. Chemphyschem. 2016. PMID: 26789782 Review.
-
Stiff-Stilbene Photoswitches: From Fundamental Studies to Emergent Applications.Angew Chem Int Ed Engl. 2020 Aug 3;59(32):13192-13202. doi: 10.1002/anie.202001031. Epub 2020 Jun 2. Angew Chem Int Ed Engl. 2020. PMID: 32222016 Free PMC article. Review.
References
-
- Pianowski Z. L., Ed., Molecular Photoswitches: Chemistry, Properties, and Applications, 2 Volume Set, Wiley, 2022.
-
- Crespi S., Simeth N. A., König B., Nat. Rev. Chem. 2019, 3, 133.
-
- Irie M., Pure Appl. Chem. 2015, 87, 617.
-
- Kortekaas L., Browne W. R., Chem. Soc. Rev. 2019, 48, 3406. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

