Aeromonas hydrophila RTX adhesin has three ligand-binding domains that give the bacterium the potential to adhere to and aggregate a wide variety of cell types
- PMID: 40243363
- PMCID: PMC12077191
- DOI: 10.1128/mbio.03158-24
Aeromonas hydrophila RTX adhesin has three ligand-binding domains that give the bacterium the potential to adhere to and aggregate a wide variety of cell types
Abstract
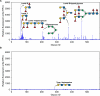
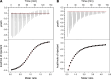
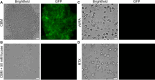
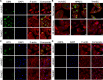
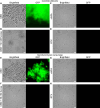
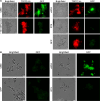
Bacteria often make initial contact with their hosts through the ligand-binding domains of large adhesin proteins. Recent analyses of repeats-in-toxin (RTX) adhesins in Gram-negative bacteria suggest that ligand-binding domains can be identified by the way they emerge from "split" domains within the adhesin. Here, using this criterion and an AlphaFold3 model of a 5047-residue RTX adhesin from Aeromonas hydrophila, we identified three different ligand-binding domains in this fibrillar protein. The crystal structures of the two novel domains were solved to 1.4 and 1.95 Å resolution, respectively, and demonstrate excellent agreement with their modeled structures. The other domain was recognized as a carbohydrate-binding module based on its beta-strand topology and confirmed by its micromolar affinity for fucosylated glycans, including the Lewis B and Y antigens. This lectin-like module, which was recombinantly produced with its companion split domain and nearby extender domain, bound to a wide variety of cells including yeasts, diatoms, erythrocytes, and human endothelial cells. In each case, 50 mM free fucose prevented this binding and may offer some protection from infection. The carbohydrate-binding module with its neighboring domains also caused aggregation of yeast and erythrocytes, which was again blocked by the addition of free fucose. The second putative ligand-binding domain has a beta-roll structure supported by a parallel alpha-helix, and the third is a homolog of a von Willebrand Factor A domain. These two domains bind to a more limited range of cell types, and their ligands have yet to be identified.IMPORTANCECharacterizing the ligand-binding domains of fibrillar adhesins is important for understanding how bacteria can colonize host surfaces and how this colonization might be blocked. Here, we show that the opportunistic pathogen, Aeromonas hydrophila, uses a carbohydrate-binding module (CBM) to attach to several different cell types. The CBM is one of three ligand-binding domains at the distal tip of the adhesin. Identifying the glycans bound by the CBM as Lewis B and Y antigens has helped explain the range of cell types that the bacterium will bind and colonize, and it has suggested sugars that might interfere with these processes. Indeed, fucose, which is a constituent of the Lewis B and Y antigens, is effective at 50 mM concentrations in blocking the attachment of the CBM to host cells. This will lead to the design of more effective inhibitors against bacterial infections.
Keywords: Aeromonas; adhesins; antimicrobial agents; binding proteins; biofilms; protein structure–function; receptor–ligand interaction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Zepeda-Velazquez AP, Gómez-De-Anda F-R, Aguilar-Mendoza LF, Castrejón-Jiménez NS, Hernández-González JC, Varela-Guerrero JA, de-la-Rosa-Arana J-L, Vega-Sánchez V, Reyes-Rodríguez NE. 2023. Bullfrogs (Lithobates catesbeianus) as a potential source of foodborne disease. J Food Prot 86:100067. doi: 10.1016/j.jfp.2023.100067 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
