The Effect of Vitamin D3 Supplementation on the Incidence of Diagnosed Dementia Among Healthy Older Adults-The Finnish Vitamin D Trial
- PMID: 40243375
- PMCID: PMC12149738
- DOI: 10.1093/gerona/glaf077
The Effect of Vitamin D3 Supplementation on the Incidence of Diagnosed Dementia Among Healthy Older Adults-The Finnish Vitamin D Trial
Abstract
Background: Some short-term vitamin D supplementation trials suggest benefits on cognitive performance, but apart from observational studies, there is little evidence whether long-term vitamin D supplementation can prevent development of dementia. We investigated whether vitamin D3 supplementation could affect the incidence of diagnosed dementia in a generally healthy population.
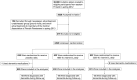
Methods: The study included 2 492 participants from the Finnish Vitamin D Trial, free of diagnosed dementia at baseline. They were randomized to placebo, 1 600 IU/d, or 3 200 IU/d of vitamin D3 arm for up to 5 years. Incident diagnoses of dementia were obtained from the national care registries.
Results: The mean age of the participants at baseline was 68.2 years and 42.8% were female. During the mean follow-up of 4.2 years, 18 participants in the placebo arm, 14 participants in the 1 600 IU/d arm (compared to placebo, hazard ratio [HR] = 0.77, 95% confidence interval [CI]: 0.38-1.55), and 13 participants in the 3 200 IU/d arm (HR = 0.72, 95% CI: 0.35-1.48) were diagnosed with dementia. Of the diagnoses, 29 were Alzheimer's disease, without statistically significant differences in the event rates between the 3 arms. Age, sex, or body mass index did not modify the effects. In the subgroup of 550 participants, the mean baseline serum 25-hydroxyvitamin D concentration was 74.8 nmol/L. After 12 months, the mean concentrations were 73.0, 99.7, and 120.4 nmol/L in the placebo, 1 600 IU/d, and 3 200 IU/d arms, respectively.
Conclusions: Five-year, medium-dose or high-dose vitamin D3 supplementation did not affect the dementia incidence in this largely vitamin D-sufficient older population.
Clinical trial registry number: ClinicalTrials.gov: NCT01463813, https://clinicaltrials.gov/ct2/show/NCT01463813.
Keywords: Cognitive decline; General population; Randomized controlled trial.
© The Author(s) 2025. Published by Oxford University Press on behalf of the Gerontological Society of America.
Conflict of interest statement
J.K.V. reports receiving travel support to vitamin D research meetings from Abiogen Pharma. C.L.-A. reports involvement in the Nordic Nutrition recommendations 2023 as a member of the Systematic Review Centre, and in European Food Safety Authority, procurement, preparatory work for the update of the tolerable upper intake levels for vitamin D, 2023. J.E.M. reports receiving grants from the National Institutes of Health during the conduct of the study, and grants from the National Institutes of Health and from Mars Edge outside the submitted work. M.U. reports receiving a grant from Orion Corp., outside the submitted work. The other authors declare no conflict.
Figures
Similar articles
-
The effect of vitamin D3 supplementation on the incidence of type 2 diabetes in healthy older adults not at high risk for diabetes (FIND): a randomised controlled trial.Diabetologia. 2025 Apr;68(4):715-726. doi: 10.1007/s00125-024-06336-9. Epub 2024 Dec 2. Diabetologia. 2025. PMID: 39621103 Free PMC article. Clinical Trial.
-
Vitamin and mineral supplementation for maintaining cognitive function in cognitively healthy people in mid and late life.Cochrane Database Syst Rev. 2018 Dec 17;12(12):CD011906. doi: 10.1002/14651858.CD011906.pub2. Cochrane Database Syst Rev. 2018. PMID: 30556597 Free PMC article.
-
Vitamin D supplementation and prevention of cardiovascular disease and cancer in the Finnish Vitamin D Trial: a randomized controlled trial.Am J Clin Nutr. 2022 May 1;115(5):1300-1310. doi: 10.1093/ajcn/nqab419. Am J Clin Nutr. 2022. PMID: 34982819 Free PMC article. Clinical Trial.
-
The effect of vitamin D3 supplementation on atrial fibrillation in generally healthy men and women: The Finnish Vitamin D Trial.Am Heart J. 2023 Oct;264:177-182. doi: 10.1016/j.ahj.2023.05.024. Epub 2023 Jun 10. Am Heart J. 2023. PMID: 37302737
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
References
-
- World Health Organization. Ageing and Health. https://www.who.int/news-room/fact-sheets/detail/ageing-and-health. Published October 1, 2024. Accessed March 14, 2025.
-
- Power MC, Mormino E, Soldan A, et al. Combined neuropathological pathways account for age-related risk of dementia. Ann Neurol. 2018;84(1):10–22. https://doi.org/ 10.1002/ana.25246 - DOI - PMC - PubMed
-
- World Health Organization. Dementia. https://www.who.int/news-room/fact-sheets/detail/dementia. Published March 15, 2023. Accessed March 14, 2025.
-
- World Health Organization. World Failing to Address Dementia Challenge. https://www.who.int/news/item/02-09-2021-world-failing-to-address-dement.... Published September 2, 2021. Accessed March 14, 2025.
-
- Gauthier S, Rosa-Neto P, Morais J, Webster C.. World Alzheimer Report 2021: Journey through the Diagnosis of Dementia. London, UK: Alzheimer’s Disease International; 2021. https://www.alzint.org/u/World-Alzheimer-Report-2021.pdf
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical