External validation of a model to predict recurrence-free and melanoma-specific survival for patients with melanoma after sentinel node biopsy
- PMID: 40243383
- PMCID: PMC12004364
- DOI: 10.1093/bjs/znaf037
External validation of a model to predict recurrence-free and melanoma-specific survival for patients with melanoma after sentinel node biopsy
Abstract
Background: Recently, a model to predict 5-year recurrence-free survival (RFS) and melanoma-specific survival (MSS) after sentinel lymph node biopsy (SLNB) was published. The aim of this study was to validate that model in a large independent international cohort.
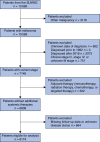
Methods: The database of the Sentinel Lymph Node Working Group (SLNWG) was analysed for patients with malignant melanoma who underwent SLNB. Patients with clinical stage III melanoma, a history of other malignancies, or receiving concomitant systemic therapies during follow-up were excluded. The model's predictive performance was evaluated using discrimination and calibration metrics in the eligible cohort. Decision curve analysis was performed to assess the clinical value of the model.
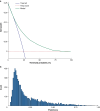
Results: The external validation cohort consisted of 6174 patients of the SLNWG from the USA, Europe, and Israel. A positive sentinel node was found in 788 patients (12.8%). The area under the time-dependent receiver operating characteristic (ROC) curve of the external validation was 0.76 (95% c.i. 0.74 to 0.77) for RFS and 0.79 (95% c.i. 0.76 to 0.81) for MSS. The model was well calibrated, as the observed 5-year survival rates aligned closely with the predicted survival rates (calibration slope of 0.98 for RFS and calibration slope of 0.99 for MSS). The model provided a net benefit versus the 'treat all' and 'treat none' strategies at the predetermined probability threshold for recurrence of 45%.
Conclusion: The model demonstrated good performance in a large heterogeneous independent cohort, emphasizing its robustness. Decision curve analysis revealed a clear net benefit of the model over a treat all strategy, highlighting its potential for clinical use.
© The Author(s) 2025. Published by Oxford University Press on behalf of BJS Foundation Ltd.
Figures


References
-
- Callender GG, Gershenwald JE, Egger ME, Scoggins CR, Martin RCG II, Schacherer CW et al. A novel and accurate computer model of melanoma prognosis for patients staged by sentinel lymph node biopsy: comparison with the American Joint Committee on Cancer model. J Am Coll Surg 2012;214:608–617 - PubMed
-
- Verver D, Rekkas A, Garbe C, van Klaveren D, van Akkooi ACJ, Rutkowski P et al. The EORTC-DeCOG nomogram adequately predicts outcomes of patients with sentinel node–positive melanoma without the need for completion lymph node dissection. Eur J Cancer 2020;134:9–18 - PubMed
-
- Stassen RC, Maas CCHM, van der Veldt AAM, Lo SN, Saw RPM, Varey AHR et al. Development and validation of a novel model to predict recurrence-free survival and melanoma-specific survival after sentinel lymph node biopsy in patients with melanoma: an international, retrospective, multicentre analysis. Lancet Oncol 2024;25:509–517 - PubMed
-
- Wong SL, Kattan MW, McMasters KM, Coit DG. A nomogram that predicts the presence of sentinel node metastasis in melanoma with better discrimination than the American Joint Committee on Cancer staging system. Ann Surg Oncol 2005;12:282–288 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

